Abstract
The objective of this study was to analyze the thermodynamic feasibility of forming nanobainite in Al-alloyed medium-Mn steels through intercritical annealing (IA) and subsequent heat treatments. The research aimed to determine the influence of IA temperature and Mn content on the stability of austenite, the Ms temperature, and the resulting bainite plate thickness (BPT). Our findings indicate that the IA temperature range of 780–860 °C effectively decreased the Ms temperature, facilitating the formation of nanobainite. The results demonstrated that a higher Mn content increases an austenite fraction during IA, thus enhancing the potential for nanobainite formation. For the 3MnNb steel, the IA temperature of 860°C was sufficient to achieve bainitic plates thinner than 100 nm, whereas the 4MnNb steel required lower IA temperatures due to its higher Mn content. The transformation kinetics was found to be faster in 3MnNb steel, with a complete transformation time of 300 min, compared to approximately 600 min for the 4MnNb steel. Dilatometric analysis confirmed that the real austenite fractions were approximately 20% higher than the ones predicted by thermodynamic simulations, indicating potential limitations of the commercial software in accurate predicting the experimental conditions. The obtained results validate the proposed heat treatment strategy for achieving nanobainitic structures in medium-Mn steels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The automotive industry is witnessing a transformative shift driven by technological advancements, environmental concerns, and changing consumer preferences. As the industry evolves, the demand for high-performance materials intensifies, especially in terms of electric vehicles (EVs) and lightweighting strategies [1,2,3]. Among these materials, advanced high-strength steels, particularly high-strength medium-manganese steels, have emerged as a promising class with the potential to revolutionize automotive engineering. This section explores the necessity for developing new advanced high-strength steels, focusing on unique advantages and applications of high-strength medium-manganese steels in the automotive industry [4]. The automotive industry faces several challenges, ranging from enhancing vehicle safety and performance to reducing environmental impact and meeting stringent regulations [1, 2]. With the growing popularity of electric vehicles, the challenges are further magnified. First is the mass reduction. Electric vehicles, in particular, demand mass reduction strategies to maximize driving range and energy efficiency [1]. Another important aspect is battery protection and safety, which require robust structural components to protect the high-voltage battery packs and ensure passenger safety during collisions [5]. High-strength steels, especially those ones with medium-manganese contents, offer an enticing solution to achieve mass savings without compromising structural integrity by good combination of strength and ductility [6]. At the same time, they exhibit excellent crash energy absorption capabilities, high formability, and design flexibility making them ideal for enhancing battery protection and vehicle safety [7]. Another type of steel for such purposes are quenched and partitioned (QP) steels containing from 0.2 to 1 mass % carbon [8]. The idea is to partially quench steel below Ms temperature to obtain from 70 to 90% of martensite and then rapidly heat it to temperatures between 300 and 500 °C [9, 10] or even 600 °C [11] to accelerate carbon diffusion into the austenite, increasing its stability. These promising steels exhibit very high mechanical properties (yield point over 1 GPa and total elongation of 15–20% [10]). However, the improvement of mechanical properties strongly depends on the retained austenite stability (its carbon enrichment and grain size) [9]. Right now, the QP treatment is modified by introducing the intercritical annealing as one of the steps [8]. In this way, it is possible to tailor the mechanical properties in a wider scope.
To ensure a high level of battery and passenger protection, with the reduction of the total mass of vehicles, high-strength phases need to be present in the microstructure of the multiphase steels. Nanobainite is a promising phase in the family of high-strength steels that have recently gained attention due to its exceptional mechanical properties [12,13,14]. It is characterized by a unique ultrafine-grained microstructure with nanoscale bainitic ferrite grains/laths leading to remarkable strength and toughness. Zhao et al. [12] presented remarkable strength levels of medium-C nanobainite steels with the tensile strength values from 2.2 to 2.6 GPa with relatively good plasticity at a level of 8%. Akram et al. [13] analyzed the possibility of obtaining the strong nanobainite within relatively short holding times. The isothermal holding time is one of the major problems during manufacturing of nanobainite steels, because in some case, it is longer than 10 h. Akram et al. [15] produced the nanobainitic steel containing 1 mass% Al with UTS of 2.1 GPa for the relatively short isothermal holding time of 2 h. Marcisz et al. [16] analyzed the influence of heat treatment parameters on the amount, phase morphology, and mechanical properties of medium-C high-Si nanobainitic steel. They reported that the nanolath, carbide-free bainite containing blocky and lath-type retained austenite achieved 2-GPa tensile strength and 10% total elongation.
While considerable research has been conducted on nanobainite formation in low alloy steels [13,14,15,16,17], there is a noticeable lack of information regarding the transformation kinetics, a role of increased manganese content, an effect of cooling rates, and the influence of other alloying elements on nanobainite formation in medium-manganese steels. These steels are characterized by some retained austenite fraction, which undergoes strain-induced martensitic transformation upon plastic deformation [4, 6]. This effect enhances mechanical properties of steel and formability of multiphase steel sheets.
Understanding the nanobainite transformation in this particular class of steels holds significant potential for advancing material science and automotive engineering. The automotive industry, driven by the pursuit of stronger yet lightweight materials, is actively seeking for new steel grades with improved performance characteristics. Medium-manganese steels, if properly engineered to form nanobainite, hold immense promise in fulfilling this demand [18, 19]. It was shown by Akram et al. [15] that Al accelerates nanobainite formation, which is important from an industrial point of view. Up to date, there are no systematic studies on the effect of Al on the nanobainite formation in multiphase medium-Mn steels. By understanding the transformation mechanisms and controlling processing parameters, automotive manufacturers can produce components with enhanced mechanical properties, leading to safer, lighter, and more fuel-efficient vehicles.
The present work is focused on thermodynamic calculations of austenite stability in two medium-Mn steels alloyed with increased Al addition. The possibility of nanobainite formation in the alloys is analyzed. The results of the analysis can enhance the knowledge on the Al-alloyed medium-manganese steels, and the ways for the generation of new types of ultrahigh-strength and ductile structures.
Material and experimental procedure
The investigation addressed two 0.17C-(X)Mn-1.6Al-0.2Si-0.2Mo-0.04Nb medium-Mn steels, where the manganese content is 3.1 and 3.6 mass.% (designated as 3MnNb and 4MnNb alloys). The chemical composition of both steels was determined using GDOS (glow discharge optical spectroscopy) after the manufacturing of the steels. Laboratory melted alloys after casting were forged and subsequently rolled in the temperature range of 1200–900 °C to a final thickness of 4,5 mm. Theoretical calculations of intercritical annealing (IA) influence on the Ms temperature were carried out using JMatPro ver. 13, general steel module [20]. It is important to note, that used software is for now, not validated for the steels with so high level of manganese. This annealing aims to form some ferrite fraction and redistribute carbon between the intercritical ferrite and austenite. The estimation of the bainite plate thickness (BPT) was carried out using an empirical model based on the thermodynamic calculations, which description is presented in the next section. The proposed heat treatment used in this analysis is shown in Fig. 1. For the purpose of this research, the simulation corresponds to the whole heat treatment (the intercritical annealing and isothermal holding in the bainite region), whereas the experimental verification is performed only for the first stage of the IA. The verification of the first crucial stage will have the strong impact on the nanobainite formation during the isothermal bainite transformation.
Since both analyzed steels have a high Ms temperature, it is impossible to form the nanobainite in them using typical full austenitization. In the work [4], the authors investigated the same steel but without the Nb addition. For the 3Mn steel, the Ms temperature was approx. 400 °C, whereas for the 5Mn, it was approx. 320 °C. It means that the analyzed steels are outside of typical nanobainitic region. That is why the two-step heat treatment is designed for the nanobainite formation during the designed thermal schedule. The purpose of the IA is to stabilize some austenite by the carbon and manganese enrichment, which should decrease the Ms temperature of the steel (actually the intercritical austenite) [8, 11]. The enrichment of the austenite is possible due to very small solubility of carbon in ferrite. Hence, it diffuses to carbides in conventional steels. In Al- or Si-containing advanced iron alloys, carbon enriches the austenite because of hampering effects of graphitizing elements on carbide precipitation [21]. Moreover, manganese as an austenite forming element is prone to diffuse into austenite. The final thermodynamic stability of austenitic phase depends on complex C and Mn contents in the austenite and its grain/lath size [22], one should note that the modeling approach only considers carbon partitioning between austenite and bainitic ferrite phases because its fundamentals are based on para-equilibrium conditions.
The IA time in this case was selected as 60 min. This time was selected to ensure full austenite transformation during isothermal holding at selected IA temperature, after which the material was cooled down at the rate of 60°/s to a designed isothermal bainite transformation (IBT) temperature. This cooling speed ensures that for 3MnNb steel, no transformation will occur before, reaching the IBT temperature. In the case of presented results, the IBT is simulated at the temperature of 240 °C (slightly higher than the Ms temperature of the 800 °C IA treatment. This temperature is the compromise between the Ms temperature and the maximum austenite fraction that can be obtained) up to 24 h, this time was selected based on the reported times used for the conventional high carbon nanobainitic steels [11,12,13], to see if bainite forms according to the simulation and how long would it take to finish the transformation at this IBT temperature.
For verifying the calculated Ms temperatures, cylindrical samples with 4 mm diameter and 10 mm length were cut from the manufactured steel plates along the rolling direction for the dilatometric investigations. The heat treatment schedules have been conducted using a BAHR DIL 805 A/D dilatometer. The experiments were conducted in vacuum; helium was used for cooling purposes. Temperature changes were measured by an S-type thermocouple welded to the central part of the sample. The dilatometry data were analyzed according to ASTM A1033-04 [23]. The applied heat treatment consisted of IA at temperatures between 780 and 860 °C (which are between Ac1 and Ac3 temperatures of the steel, which are 717 and 1005 °C), carried out for 60 min. This time was selected as it ensures full austenitic formation at each IA temperature. Next, samples were cooled at the rate of 60 °C/s to room temperature (RT) to determine the real Ms temperature after the different IA. After the heat treatment completion, the samples were cut perpendicularly to their length in half and prepared according to typical metallographic procedures: grinding up to SiC paper 2000; polished using 3, 1-µm diamond pastes; and finally etched using nital. The investigation of the microstructure after IA was conducted using Zeiss AXIO Observer Z1m light microscopy. The purpose of this investigation was to determine the initial microstructure composition, which at latter work will be subjected to the IBT.
Modeling the bainite plate thickness
The bainitic transformation is an incomplete reaction in the investigated thermal conditions, which implies that the bainitic ferrite sub-unit growth is inhibited before the austenite reaches its para-equilibrium composition [24]. Austenite transformation to bainite is assumed to occur in the diffusionless growth manner, namely, the bainite sub-unit growth swiftly to a finite volume just after its nucleation [25]. The model proposed by Azuma et al. [26] that enables calculation of bainite plate thickness is applied in the current work. Accordingly, to calculate BPT in µm, the following formula is used:
where T is a temperature in Celsius; and \({\Delta G}_{\text{max}}^{\gamma \to \alpha }\) is a driving force for bainitic ferrite nucleation under para-equilibrium with austenite, that can be calculated using a thermodynamic model developed by Peet and Bhadeshia in work [27] accordingly to a parallel tangent construction method. The austenite strength (Sγ) parameter in Eq. (1) is determined in MPa based on the empirical equation proposed by Azuma [26] in the following form:
where the symbols for the individual elements indicate their concentrations in mass.%. Equations (1) and (2), as well as the procedures extracted from the MAP_STEEL_MUCG83 [27] Fortran source code for calculating the \({\Delta G}_{\text{max}}^{\gamma \to \alpha }\) parameter, were implemented in the C+ + programming language as a complex computer program. This computational tool is dedicated to performing numerical calculations and predicting BPT according to temperature conditions and the chemical composition of the austenite.
Results and discussion
Theoretical calculations of austenite stability and bainite plate thickness
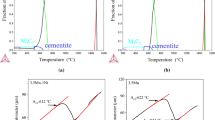
The first stage of the analysis was to determine the effect of the IA temperature on the austenite stability (Ms temperature). According to the results of JMatPro calculations presented in Fig. 2, it can be seen that at the highest annealing temperature (860 °C), the Ms temperature is about 300 °C for 3MnNb steel and 290 °C for 4MnNb steel. However, when the IA temperature is lowered, the Ms temperature drops to ~ 170 °C for both steels. The drop in Ms temperature is caused by the increasing content of ferrite in the microstructure, as it is shown in Fig. 3. Since only a small amount of carbon and manganese can be dissolved in ferrite, their excess diffuses into austenite [28, 29]. The more ferrite in the microstructure, the more austenite is enriched in both elements increasing its stability (corresponding to the Ms decrease). This means that the IA temperature between 780 and 860 °C is enough to decrease the Ms temperature to a range, where the formation of nanobainite structures should be possible. Another important aspect of the austenite stability is its size. When the austenite size is higher (higher IA temperature), the stability of it decreases, and it is more prone to martensite transformation. Matsuoka et al. [30] analyzed the austenite grain size impact on the martensite transformation in austenitic stainless steel. They showed that when the austenite grain size was 20 µm, approx. 30% of martensite was formed. However, when the austenite grain size decreased to 1 µm, only 3% of martensite was formed. According to Inokuti et al. [31], smaller austenite grains exhibit the lower amount of preferable places for martensite nucleation, increasing its stability. Moreover, Brofman et al. [32] analyzed the stability of austenite in Fe-27Ni-0.025C steel. They explained that the smaller austenite is more stable because of the higher dislocation density and dislocation pill-up is higher in smaller grains. This hampers the martensite transformation, as the austenite is more resistant to local plastic deformation. However, as this aspect is not taken in account by the JMatPro software, it was not analyzed in case of this manuscript.
According to the austenite and ferrite amount calculations during the IA for both steels (see Fig. 2 and Table 1), it can be seen that the highest amount of austenite is present at 860 °C. For the 3MnNb steel, the amount of the austenite is 54%, whereas for 4MnNb steel, it is 63%. When the lowest temperature is applied, the austenite amount decreases to 33 and 40%, respectively. This is the result of the manganese impact on intercritical temperatures. A higher manganese content results in lower Ac1 and Ac3 temperatures because it is an austenite stabilizer. Hence, if we compare two different manganese contents, the steel with its higher level will have a higher austenite fraction at the same IA temperature. This is the result of the shift of the ferrite+austenite region, when the critical temperatures are lower. As the example of 800 °C, the Ac3 temperature of the 3MnNb is equal to 1005 °C (according to the dilatometry results); however, the Ac3 temperature for the 4MnNb steel is 970 °C. Therefore, for the 4MnNb, 800 °C is closer to the Ac3 temperature because there is a higher amount of the austenite at 800 °C (at Ac3, an austenite level is approx. 100%). Kucerova et al. [33] showed that when a Mn content of the steel was increased from 1.5 to 3.0%, the Ac3 temperature decreased from 950 to 900 °C. Similar results were presented by Zhan et al. [34]. They showed that the Ac1, Ac3, Ms, and Mf temperatures decrease with increasing a manganese content. The difference between the temperatures for 2Mn and 5Mn steels was: 32 °C for Ac3, 33 °C for Ac1, 120 °C for Ms, and 125 °C for Mf temperatures. The amount of austenite is an important factor because it directly influences the maximum amount of the nanobainite that could be formed. The results show that it is better to use a higher IA temperature to form more nanobainite. Moreover, the higher manganese content should result in a larger austenite fraction at the same IA temperature. However, it is important to discuss the slight balance that occurs when increasing the IA temperature. When the IA temperature is increased, the austenite fraction indeed increases, but the carbon content within the austenite decreases due to the redistribution of carbon between the austenite and ferrite phases. This decrease in carbon content in austenite affects its stability, leading to an increase in the Ms temperature. Therefore, while a higher IA temperature results in a larger austenite fraction, the decreased carbon content can potentially lead to a less stable austenite phase, which might transform into martensite during cooling. This delicate balance between austenite fraction and carbon content must be carefully managed to optimize the formation of nanobainite. A higher austenite fraction at elevated IA temperatures is beneficial for forming more nanobainite, but only if the carbon content remains sufficiently high to stabilize the austenite phase during subsequent cooling. The Ms temperature is crucial in this context, as a higher Ms temperature can promote the formation of martensite instead of bainite, thus affecting the final microstructure. Therefore, the choice of IA temperature must consider both the austenite fraction and its carbon content to ensure the successful formation of nanobainite. Furthermore, one can see that the thermodynamic equilibrium between both phases (50% ferrite and 50% austenite) occurs at a lower temperature for the 4MnNb steel, what is related to the higher manganese content. Haupt et al. [35] presented results of the influence of intercritical annealing time on the austenite fraction in 12% Mn alloy. According to their results, when Mn and C levels in the austenite increase, its stability increases too (which could result in less amount of the austenite transformed into the martensite in the microstructure of the steel). Here, we could analyze two ways. One is the assumption of fully homogeneous chemical composition of the austenite. In this case, the increase in the Mn and C contents increases its stability and depending on the amount of these elements, the austenite will or not will change into the martensite. However, this is not the case for medium-manganese steels, where a local microsegregation is always present [36]. That is why in the second way, we assume that there are regions of austenite with higher and lower Mn and C levels. This means that the enriched austenite will not transform into the martensite, and the area of lower level of these elements will. Moreover, a manganese content strongly influences the Ac1 (start of austenite transformation) and Ac3 (finish of austenite transformation) temperatures, which characterize the IA region. Dykas et al. [37] analyzed the influence of different manganese and carbon contents on continues cooling transformation diagrams in medium-manganese steels. They reported that both temperatures decrease from 702 and 947 °C (for the steel containing 2%Mn), respectively, to 557 and 703 °C in case of 10%Mn steel. Their results correspond well to the calculations presented in Fig. 3.
The results of the bainite plate thickness calculations are presented in Fig. 4. To determine what is nanobainite, the limit of 100 nm was applied, as defined in the work of Bhadeshia, who characterized nanobainite by the presence of bainitic ferrite plates with a thickness below this threshold [38]. If the BPT is lower than 100 nm, it means the formation of nanobainite. One can easily interpret, which intercritical annealing temperature guarantees the formation of the nanobainite after a heat treatment near the Ms temperature. Taking into account that for nanobainite, the 100-nm plate thickness is the limit, the nanobainite could not be obtained at the highest annealing temperature (860 °C) in case of 4MnNb steel. According to the model for the 3MnNb and 4MnNb steels, the bainite formation is not possible both at 800 and 780 °C. Moreover, the effect of Mn on the BPT is also visible. Manganese plays a crucial role in the behavior of medium-Mn steels and significantly influences several aspects of their microstructure and mechanical properties. Mn is an austenite stabilizer, which means that it lowers the Ms temperature. By reducing the Ms temperature, Mn promotes the retention of austenite at lower temperatures, which is essential for achieving the desired microstructure in medium-Mn steels. Mn affects the free energy of the phases involved in the transformation processes. It reduces the Gibbs free energy difference between austenite and ferrite, thereby stabilizing austenite [39]. This stabilization is critical in controlling the phase transformations and ensuring the retention of austenite during cooling. Mn contributes to the solid solution strengthening of austenite. The presence of Mn atoms in the austenite lattice increases its strength by impeding dislocation movement. This strengthening effect is particularly significant in medium-Mn steels, where higher Mn contents can lead to improved mechanical properties such as increased tensile strength and hardness [4]. Additionally, the increased strength of austenite can affect the bainite plate thickness (BPT) by influencing the growth kinetics of bainitic ferrite [24]. The steel with the higher manganese level shows the higher BPT compared to the steel with the lower Mn content at the same IA temperature. This means that lower IA temperatures need to be used before the nanobainite formation because it determines the lowest possible temperature, which can be used for the bainite formation. Akram et al. [15] reported that a decrease in the isothermal bainite transformation temperature decreases the BPT too. The BPT decreased from 54 to 34 nm with decreasing the isothermal holding temperature from 350 to 265 °C.
Another important aspect of the nanobainite formation is its transformation kinetics. According to the JMatPro simulations (Fig. 5), it can be seen that at the lowest IA temperature, the maximum amount of bainite that could be formed at the isothermal holding temperature of 240 °C is around 50%. The amount of it increases together with increasing the IA temperature, what is the result of higher austenite amount available for the transformation (Table 1). The opposite effect is visible at the IA temperature of 840°C. This is due to the fact that at this IA temperature, the bainite transformation at 240 °C crossed the Ms temperature. This resulted in the partial martensite formation decreasing the maximum available amount of austenite. This shows that the isothermal bainite transformation should be selected at the temperature slightly higher than the Ms. According to the simulation, the bainitic transformation at 240 °C should finish after 5 h. Prolonging the time of the isothermal holding results in a slight decrease in the bainite fraction.
The 4MnNb steel exhibits the different transformation kinetics compared to the 3MnNb steel. First of all, the simulations show that more bainite could be formed in 4MnNb steel at the same isothermal temperature (Fig. 5). Moreover, the time for the transformation to start and finish is much longer compared to the 3MnNb steel. The transformation should start after ~ 40 min and finish after ~ 11 h. This shows that manganese decreases the driving force for the bainite transformation and requires much longer time. Such effect was reported by Morawiec et al. [39] in the study concerning the effect of three Mn contents on the bainite transformation kinetics. According to the results, the times to start and finish bainitic transformation were strongly prolonged with increasing the Mn content from 3.1, through 3.6 to 5 mass%. For the lowest Mn concentration, the transformation was finished after 500 s, whereas for the 5Mn steel, it was not even started after 3 h. This confirms that manganese decreases the driving force for the austenite to bainite transition. Moreover, Kral et al. [40] analyzed the carbon and manganese diffusion in Fe–C–Mn alloys. According to their results, Mn decreases the carbon diffusion in iron alloys. The obtained results are in accordance with earlier papers concerning the bainite formation [36, 39]. According to our previous work [39] on the chemical driving force for austenite to ferrite formation, the increase in a Mn content from 3 to 5% resulted in the Gibbs free energy change from − 1142 to − 781 J/mol. Guo et al. [41] indicated the inhibiting effect of manganese on the bainite transformation kinetics through the fact that not only the finishing transformation time was longer but the extension of the incubation time was also noticed.
Dilatometric and microstructural results
For the verification of the Ms calculation results and the amount of austenite, the dilatometric analysis of IA (which is the first step of heat treatment) was carried out. The dilatometric curves presenting the relative change in length (RCL) during cooling to RT after the IA for 3MnNb steel are presented in Fig. 6. According to the experimental results, the calculated Ms temperature is close to the real one. The comparison of both temperatures for the 3MnNb steel is presented in Table 2. One can see that a decrease in IA temperature results in a lower Ms temperature. However, one interesting effect was registered during the analysis (red circle in Fig. 6).
During the martensite formation, the change in a curve slope is visible, which corresponds to the two-stage martensite formation. This is presumably the result of the inhomogeneity of austenite after the IA. This effect is reported for medium-Mn steels and corresponds to austenite regions of higher and lower thermal stability, resulting from the chemical microsegregation [36]. Firstly, the austenite of lower stability undergoes martensite transformation, then the highly stable austenite is transformed at a lower temperature.
Since the nanobainite amount in steel is directly related to the amount of austenite before the IBT, dilatometric results were used to determine the real austenite amount after the IA. For this purpose, the analysis of the RCL of quenched and IA-treated steel was carried out. For this analysis, the ΔRCL was determined (the difference of RCL and the beginning and finish of martensite transformation). The ΔRCL of the quenched sample representing 100% of austenite is − 0.22%. For the IA treatment, the ΔRCL depends on the amount of the austenite transforming into martensite. In general, more austenite undergoing the transformation results in a higher difference of RCL at the start and finish of the martensite transformation. Then using the proportion approach, between quenched and IA results, it is possible to determine the amount of austenite that was subjected to martensite transformation. The results of the analysis are listed in Table 3.
According to the results, it is clear that the real amount of austenite is higher compared to the prediction made by JMatPro software. At 860 °C and 780 °C, the calculations for 3MnNb steel show the austenite amount at the level of 54 and 33%, respectively. However, the dilatometric results reveal that the amount of austenite is ~ 20% higher compared to the calculations. This means that in reality, more austenite could undergo nanobainite transformation during the designed heat treatment. This discrepancy raises important questions about the accuracy of the thermodynamic simulations. Thermodynamic simulations typically assume equilibrium conditions, where the formed austenite fraction should theoretically represent the highest possible value for each temperature. The fact that our experimental results show a higher austenite fraction under non-equilibrium conditions suggests that the model may not fully capture the complexities of the actual process. One possible explanation for this difference is the kinetics of the phase transformations during the IA process. The simulations might not account for certain kinetic factors, such as the rate of carbon diffusion or the presence of non-equilibrium phases, which can lead to higher austenite fractions in practice.
The results are in accordance with the microstructural analysis presented in Fig. 7. The microstructure is composed of two phases (white—ferrite and brown—martensite). According to the microstructures, at 780 °C (Fig. 7a), 800 °C (Fig. 7b), and 840 °C (Fig. 7c), a high amount of ferrite (white areas) laths are present (according to Table 3, the amount of austenite is between 50 and 59%). On the other hand, at 860 °C (Fig. 7d), the martensite laths (brown areas) have dominated the structure of the steel because this phase occurs preferentially (73% of austenite undergoes the martensitic transformation). The lath-like morphology of austenite and ferrite is typical for this type of steel, which is the result of the initial martensite microstructure. Without the deformation, both austenite and ferrite inherit the morphology from parent phase.
Conclusions
Results presented in the study aimed at the thermodynamic analysis of the possibility of nanobainite formation in Al-alloyed medium-Mn steels. It was proved that there is the possibility of nanobainite formation under some conditions of intercritical annealing. The following conclusions can be drawn concerning the proposed heat treatment:
-
The IA in a range of 780–860 °C allows for the Ms decrease to the necessary level in terms of nanobainite formation. The higher Mn content provides a higher austenite fraction during the IA at the same temperature (approximately 7–10% higher amount of austenite).
-
4MnNb steel exhibits slightly different Ms temperatures compared to 3MnNb steel. This is the result of higher Mn content, which is an austenite stabilizer. This means that increasing a Mn content in the alloy provides the higher nanobainite amount.
-
4MnNb steel shows a higher BPT compared to the 3MnNb steel at the same IA temperature. This means that lower IA temperatures should be used to produce nanobainite in the 4MnNb steel. For 3MnNb steel, the IA temperature of 860°C is enough to obtain a thickness of bainitic plates lower than 100 nm. For 4MnNb steel, lower temperatures are necessary to obtain the same BPT (at the expense of the austenite amount).
-
The nanobainite formation kinetics is faster in 3MnNb steel. The total time to finish the nanobainite formation in 3MnNb steel is 300 min, whereas for the 4MnNb steel, it is approximately 2 times longer.
-
The analysis of austenite fraction at different IA temperatures shows that the highest possible austenite fraction in 3MnNb steel allowing to form nanobainite is ca. 60%. The remaining 40% of the structure will be ferrite.
-
The formation of nanobainite is influenced by the delicate balance between the austenite fraction and its carbon content during intercritical annealing. Higher IA temperatures increase the austenite fraction but decrease its carbon content.
-
The experimental results suggest that commercial thermodynamic models may have limitations in predicting austenite fractions under specific experimental conditions, indicating the need for further refinement or supplementary experimental data to enhance the accuracy of such predictions.
References
Wang P, Bai Y, Fu C, Lin C. Lightweight design of an electric bus body structure with analytical target cascading. Front Mech Eng. 2023. https://doi.org/10.1007/s11465-022-0718-y.
Del Pero F, Berzi L, Antonacci A, Delogu M. Automotive lightweight design: simulation modeling of mass-related consumption for electric vehicles. Machines. 2020. https://doi.org/10.3390/machines8030051.
Tsirogiannis ChE, Stavroulakis EG, Makridis S. Design and modeling methodologies of an efficient and lightweirht carbon-fiber reinforced epoxy monocoque chassis, suitable for an electric car. Mater Sci Eng Adv Res. 2017. https://doi.org/10.24218/msear.2017.21.
Grajcar A, Zalecki W, Burian W, Kozłowska A. Phase equilibrium and austenite decomposition in advanced high-strength medium-Mn bainitic steels. Metals. 2016. https://doi.org/10.3390/met6100248.
Xu J, Ma J, Zhao X, Chen H, Xu B, Wu X. Detection technology for battery safety in electric vehicles: a review. Energies. 2020. https://doi.org/10.3390/en13184636.
Grajcar A, Skrzypczyk P, Kozłowska A. Effects of temperature and time of isothermal holding on retained austenite stability in medium-Mn steels. App Sci. 2018. https://doi.org/10.3390/app8112156.
Shen F, Wang H, Liu Z, Liu W, Konemann M, Yuan G, Wang G, Munstermann S, Lian J. Local formability of medium-Mn steel. J Mater Proc Tech. 2022. https://doi.org/10.1016/j.jmatprotec.2021.117368.
Liu S, Xiong Z, Guo H, Shang C, Misra RDK. The significance of multi-step partitioning: processing-structure-property relationship in governing high strength-high ductility combination in medium-manganese steels. Acta Mater. 2017. https://doi.org/10.1016/j.actamat.2016.10.067.
Goune M, Aoued S, Danoix F, Geandier G, Poulon-Quintin A, Hell JC, Soler M, Allain SYP. Alloying-element interactions with austenite/martensite interface during quenching and partitioning of a model Fe-C-Mn-Si alloy. Scr Mater. 2019. https://doi.org/10.1016/j.scriptamat.2018.11.012.
Kim HJ, Kwon MH, Gu G, Lee SJ, Suh DW. Quenching and partitioning (Q&P) processed medium Mn steel starting from heterogeneous microstructure. Materialia. 2020. https://doi.org/10.1016/j.mtla.2020.100757.
Arlazarov A, Bouaziz O, Masse JP, Kegel F. Characterization and modeling of mechanical behavior of quenching and partitioning steels. Mater Sci Eng A. 2015. https://doi.org/10.1016/j.msea.2014.10.034.
Zhao J, Guo K, He YM, Wang YF, Wang TS. Extremely high strength achievement in medium-C nanobainite steel. Scrip Mater. 2018. https://doi.org/10.1016/j.scriptamat.2018.04.005.
Akram M, Palkowski H, Soliman M. High-strength low-cost nano-bainitic steel. J Mater Eng Perform. 2020. https://doi.org/10.1007/s11665-020-04771-4.
Akram M, Palkowski H, Soliman M. Nanobainite generated in low- and medium-carbon steels via an economical alloying strategy. Steel Res Inter. 2021. https://doi.org/10.1002/srin.202100575.
Akram M, Soliman M, Palkowski H. Nano-bainitic steels: acceleration of transformation by high aluminum addition and its effect on their mechanical properties. Metals. 2021. https://doi.org/10.3390/met11081210.
Marcisz J, Garbarz B, Janik A, Zalecki W. Controlling the content and morphology of phases constituents in nanobainitic steel containing 0.6%C to obtain the required ration of strength to plasticity. Metals. 2021. https://doi.org/10.3390/met11040658.
Kumar A, Singh A. Mechanical properties of nanostructured bainitic steels. Materialia. 2021. https://doi.org/10.1016/j.mtla.2021.101034.
Lee JY, Kim M, Lee YK. Design of high strength medium-Mn steel using machine learning. Mater Sci Eng A. 2022. https://doi.org/10.1016/j.msea.2022.143148.
Singh MK, Verma AK, Kumar A. Microstructure and mechanical properties of medium manganese steels. Mater Today Proc. 2022. https://doi.org/10.1016/j.matpr.2022.01.195.
Sente software Ltd. (2005) A collection of free downloadable papers on the development and application of JMatPro. http://www.sentesoftware.co.uk/biblio.html
Kaar S, Krizan D, Schneider R, Sommitsch C. Impact of Si and Al on microstructural evolution and mechanical properties of lean medium manganese quenching and partitioning steel. Steel Res Inter. 2020. https://doi.org/10.1002/srin.202000181.
Xiao H, Zhao G, Xu D, Cheng Y, Bao S. Effect of microstructure morphology of Q&P steel on carbon and manganese partitioning and stability of retained austenite. Metals. 2022;12(10):1613.
ASTM A1033–04. Standard practice for quantitative measurement and reporting of hypoeutectoid carbon and low-alloy steel phase transformations; ASTM International: West Conshohocken [Internet]. 2004 [cited 2020 Jul 25]. Available from https://www.astm.org/
Bhadeshia HKDH. Bainite in steels: theory and practice. CRC Press; 2019. https://doi.org/10.1201/9781315096674.
Rees GI, Bhadeshia HKDH. Bainite transformation kinetics Part 1 modified model. Mater Sci Tech. 1992. https://doi.org/10.1179/mst.1992.8.11.985.
Azuma M, Fujita N, Takahashi M, Senuma T, Quidort D, Lung T. Modelling upper and lower bainite transformation in steels. ISIJ Inter. 2005. https://doi.org/10.4028/www.scientific.net/MSF.426-432.1405.
Peet M., Bhadeshia H.K.D.H. MAP_STEEL_MUCG83, Materials Algorithms Project (MAP), Department of Materials Science and Metallurgy, University of Cambridge, U.K., https://www.phase-trans.msm.cam.ac.uk/map/steel/programs/mucg83.html
Jiang D, Carter AE. Carbon dissolution and diffusion in ferrite and austenite from first principles. Phys Rev B Cond Matter. 2003. https://doi.org/10.1103/PhysRevB.67.214103.
Mueller JJ, Hu X, Sun X, Ren Y, Choi K, Barker E, Speer JG, Matlock DK, De Moor E. Austenite formation and cementite dissolution during intercritical annealing of a medium-manganese steel from a martensite condition. Mater Des. 2021. https://doi.org/10.1016/j.matdes.2021.109598.
Matsuoka Y, Iwasaki T, Nakada N, Tsuchiyama T, Takaki S. Effect of grain size on thermal and mechanical stability of austenite in metastable austenitic stainless steel. ISIJ Inter. 2013. https://doi.org/10.2355/isijinternational.53.1224.
Inokuti Y, Cantor B. Overview 15 the microstructure and kinetics of martensite transformation in splat-quenched Fe and Fe Ni alloys-II. Fe Ni alloys Acta Metal. 1982. https://doi.org/10.1016/0001-6160(82)90214-0.
Brofman PJ, Ansell GS. On the effect of fine grain size on the Ms temperature on Fe-27Ni-0.025C alloys. Metal Trans A. 1983;14:1929–31.
Kucerova L, Jirkova H, Volkmannova J, Vrtacek J. Effect of aluminium and manganese content on the microstructure development of forged and annealed TRIP steel. Manufac Tech. 2018. https://doi.org/10.21062/ujep/146.2018/a/1213-2489/MT/18/4/605.
Zhan Z, Shi Z, Wang Z, Lu W, Chen Z, Zhang D, Chai F, Luo X. Effect of manganese on the strength-toughness relationship of low-carbon copper and nickel-containing hull steel. Mater. 2024. https://doi.org/10.3390/ma17051012.
Haupt M, Dutta A, Ponge D, Sandlobes S, Nellessen M, Hirt G. Influence of intercrtical annealing on microstructure and mechanical properties of a medium manganese steel. Proc Eng. 2017. https://doi.org/10.1016/j.proeng.2017.10.942.
Morawiec M, Ruiz-Jimenez V, Garcia-Mateo C, Jimenez JA, Grajcar A. Study if the isothermal bainitic transformation and austenite stability in an advanced Al-rich medium-Mn steel. Arch Civ Mecha Eng. 2022. https://doi.org/10.1007/s43452-022-00475-9.
Dykas J, Samek L, Grajcar A, Kozłowska A. Modelling of phase diagrams and continuous cooling transformation diagrams of medium manganese steels. Symmetry. 2023. https://doi.org/10.3390/sym15020381.
Bhadeshia HKDH. Nanostructured bainite. Proc R Soc A. 2010. https://doi.org/10.1098/rspa.2009.0407.
Morawiec M, Opara J, Garcia-Mateo C, Jimenez JA, Grajcar A. Effect of Mn on the chemical driving force and bainite transformation kinetic in medium-manganese alloys. J Therm Anal Calorim. 2022. https://doi.org/10.1007/s10973-022-11664-2.
Kral L, Million B, Cermak J. Diffusion of carbon and manganese in Fe-C-Mn. Def Diff Form. 2007. https://doi.org/10.4028/www.scientific.net/DDF.263.153.
Guo H, Zhou P, Zhao AM, Zhi C, Ding R, Wang JX. Effects of Mn and Cr contents on microstructures and mechanical properties of low temperature bainitic steel. J Iron Steel Res Inter. 2017. https://doi.org/10.1016/S1006-706X(17)30042-0.
Acknowledgements
M. Morawiec acknowledges the financial support of the National Science Center through the grant no. 2021/41/N/ST8/03371.
Author information
Authors and Affiliations
Contributions
[Mateusz Morawiec] and [Adam Grajcar] contributed to the study conception and design. Material preparation, data collection, and analysis were performed by [Mateusz Morawiec] and [Jarosław Opara]. The first draft of the manuscript was written by [Mateusz Morawiec]. Initial review was carried out by [Adam Grajcar] and [Jarosław Opara]. All authors read and approved the final manuscript.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morawiec, M., Opara, J. & Grajcar, A. Nanobainite formation in high-Al medium-Mn steels: thermodynamic approach. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13441-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13441-9