Abstract
Non-isocyanate polyurethanes (NIPUs) are considered as a class of environmentally-safe polymers that show promising properties, such as chemical and mechanical resistance. An important feature that may limit some important applications is the thermal degradation behavior of NIPUs and their composites and hybrids. Hence, this article comprehensively reviews recent developments in these materials groups, focusing on the thermal stability and degradation routes. Influence of urethane linkage vicinity, molar mass and ratio of carbonate and amine components, and chemical structure on NIPU thermal degradation behavior was discussed. The onset temperature of degradation was found to be mainly influenced by urethane bonds concentration and crosslinking density of NIPU material. Chain length of amine component has also a significant impact on the thermal degradation profile. The incorporation of bio-sourced and nano-scaled additives (carbon- and silica-based nanoparticles) and their impact on thermal stability of NIPU matrix was analyzed, too, and future outlooks were given.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The importance of studying the thermal degradation pathways of polymeric materials is unquestionably. The course of the degradation process, including determination of the thermal stability parameters (such as Tonset), qualitative and quantitative analysis of volatiles formed, as well as solid residue yield, composition and microstructure are essential information when new polymers are obtained. Such knowledge allows to design new effective thermal stabilizers (when required), but also contribute to the development of efficient waste utilization protocols [1]. Studying polymers thermal stability is of crucial importance for new bio-based and environmentally-friendly materials, in which raw materials from natural sources are applied or toxic substrates are eliminated in the synthesis stage [2,3,4]. Among such materials growing attention has been paid recently to non-isocyanate polyurethanes (NIPUs) that are produced without the use of toxic isocyanates.
In general, polyurethanes (PUs) are a class of highly complex polymers with urethane linkage that can be synthesized from various di- or polyisocyanates and diols. Thermal stability and degradation routes depend on the PU composition and microstructure. In the past decades, significant number of works described the thermal degradation processes in polyurethanes, both in inert and oxidative atmosphere [5,6,7,8,9,10,11,12]. The thermal degradation paths upon different conditions, kinetic analysis, characteristics of low-molecular-mass volatile products and char residue, and the structure-thermal properties relationships were proposed for various types of polyurethanes [13, 14]. It is generally agreed that thermal degradation of PUs involves three main degradation paths: dissociation, formation of primary and secondary amines and transesterification—Fig. 1. Final products of decomposition include carbon dioxide, amines, alcohols and olefins [15,16,17].
Non-isocyanate polyurethanes (NIPUs) have been developed as an answer for growing concerns about hazardous isocyanates used in the synthesis of conventional polyurethanes. NIPUs can be obtained by polycondensation [18, 19], polyaddition [20, 21], rearrangement [22] and ring-opening reaction [23]. Although a variety of methods of NIPU synthesis was developed, one of it attracted greater attention, namely polyaddition reaction between cyclic carbonates and diamines. Due to possible green paths of obtaining cyclic carbonates using different glycidyl-substituted compounds originated from natural resources, such method mostly applied to produce NIPU material [23,24,25,26]. In comparison with conventional polyurethanes, NIPU obtained with this method has one important difference, that is, the presence of hydroxyl groups next to urethane bonds. Polyhydroxyurethanes (PHUs) are described in the literature as polymers characterized by higher chemical stability then PU. It was also found that they may show interesting thermal behavior in, e.g., glass transition area, due to structural differences in the urethane region vicinity [27, 28].
The aim of this work is to present thermal degradation behavior of non-isocyanate polyurethanes, including discussion on the influence of preparation methods, as well as components and additives applied on the thermal decomposition of NIPUs.
Urethane linkage vicinity vs NIPU thermal degradation behavior
Various synthetic paths lead to NIPU with different chemical structures in the vicinity of urethane bonds—Fig. 2. For most of polyurethane materials, this region is first during pyrolysis that undergoes thermal decomposition. Therefore, it seems reasonable to investigate the impact of the method used to obtain NIPU on its thermal degradation behavior.
Hence, Cornille et al. [29] compared the thermal properties of NIPUs synthesized from poly(propylene oxide) biscarbonate, trimethylol-propane triscarbonate with aliphatic amine and conventional PU foams. The investigated NIPU materials were characterized by high thermal stability with onset temperature of ca. 200 °C; however, commercial PU based on unsaturated polyol with ether bonds and aromatic isocyanates exhibited much higher thermal stability of 250 °C. It was stated by authors that such difference is due to presence of hydroxyl group in the vicinity of urethane bonds in NIPU that may weaken their stability upon heating. Similar hypothesis was proposed in other works [30,31,32,33]. In contrary, beneficial effect of hydroxyl group on the onset temperature of first step of degradation in NIPU was postulated by Poussant et al. [27]. Authors synthesized NIPUs based on carbonated soybean oil and fatty acid originated amines. The temperature at 5% mass loss for all of prepared samples was ca. 350 °C, much higher than for conventional PUs. Possible explanation of such phenomena could be the hydrogen bonding formed with hydroxyl group in the vicinity of urethanes. Analogous explanation of NIPUs higher thermal stability was proposed in Ref. [28]. Hydrogen bonds are known for their abilities to enhance the thermal and mechanical properties in various biological systems; moreover, they facilitate formation of thermally-robust crystalline phase. Hydrogen bonds in NIPU and their stability were studied by Zhang and coworkers [34]. Authors used variable temperature FTIR spectroscopy to investigate carbonyl (1500–1700 cm−1) and amide (3000–3500 cm−1) regions in segmented poly (amide-hydroxyurethane) copolymers. It was observed that while heating up to 195 °C, intensities of bands corresponding to hydrogen bonds decrease, for the benefit of the formation of bands from free carbonyl and hydroxyl groups—Fig. 3. This study shows that hydrogen bonds, although play crucial role for macrochains mobility, are unstable at high temperatures and therefore, shall play a minor role in the stability of NIPU at temperatures higher than 200 °C.
VT FTIR spectra of NIPU (a) in the carbonyl region and (b) in the amide region. Reprinted with permission from RSC [34]
Thermal degradation of urethane bonds in both NIPU and PU materials foams has been studied by Cornille et al. using TGA/FTIR technique [29]. It was shown that during NIPUs degradation, the release of CO2 occurs during all degradation steps, although there is a decreased formation of carbon dioxide at higher temperatures. Interestingly, NIPUs thermodegradation causes higher release of CO2 in comparison with conventional polyurethanes. It was attributed to the higher amount of urethane linkages in NIPUs in comparison with PUs. As shown by the Py-GC/MS study done by our research group [35], the main products of decomposition of urethane groups in NIPUs were ammonia and carbon dioxide. Therefore, the concentration of the produced gases in the first stage of degradation may provide some information about the thermal stability in the initial stage. Noteworthy, both studies showed that the course of the degradation process of both PU and NIPU is analogous, with dissipation of urethane moieties in the first degradation step that leads to the formation of carbon dioxide and polyols. However, as it was shown [35], the decomposition of the NIPUs matrix might also produce more complex compounds, such as glutamic acid derivatives, acrolein cyclic amides or even isocyanates—pyrolysis of NIPUs where 1,4-diaminobutane (DAB) was used as a chain extender resulted in formation of tetramethylene diisocyanate at temperatures above 350 °C.
The presence of moieties other than hydroxyl group in the vicinity of urethane linkage can also influence the thermal stability of NIPU. Monie et al. [36] studied NIPU foams with addition of thioether as a regioselective nucleophile in combination with amines. The presence of hydroxythioether group in the vicinity of urethane linkage caused an increase in the temperature of 5% mass loss from 222 to 289 °C with increasing ratio of hydroxythioethers in polymer chain.
The works presented so far concerned NIPU obtained by the polyaddition method. In the case of polycondensation, there are no additional hydroxyl groups close to the urethane group. The only factor which presence in the urethane vicinity could affect the stability of the bond is the number of methylene groups between urethane groups. Duval et al. [37] investigated the influence of amines and diols with a different methylene chain length on thermal stability of NIPU. It was shown that with an increase in chain length of amine and diol, temperature of 5% mass loss increases as well. Those results are in accordance with chain extender length influence on thermal stability in conventional segmental polyurethanes [38,39,40].
Influence of components on the thermal stability of NIPU
In the conventional PUs, the thermal stability strongly depends on the raw materials used [41,42,43,44]. Similarly, in NIPUs, the structure and ratio of used carbonates and amines significantly impacts on the degradation route. It is important to highlight that in such a complex systems like NIPUs, it is quite hard to determine the (individual) influence of each factor on the polymer thermal stability especially that these factors can act together or separately in a complex environment and at elevated temperature.
Carbonate components
As it was mentioned before, urethane linkage undergoes thermal decomposition in the first place in both NIPUs and PUs. This may lead to the conclusion that the urethane groups constitute specific weak points in the polymer chain subjected to pyrolysis. Therefore, the concentration of these groups strongly influences polymer’s thermal stability. Since carbonates are one of the main components forming urethane bonds, their structure and mass ratio influence the temperature at the beginning of pyrolytic degradation. One of the factors that determine the number of urethane groups formed in NIPU is the length of the carbonate chain, or in other words its molecular mass. In the work by Asemani et al. [32], two types of cyclic carbonates with different length of methylene chain were reacted with the same substrates. TG measurements showed that shorter cyclic carbonate with lower percentage of urethanes -13.2 mass%, exhibit lower thermal stability then cyclic carbonate with longer chains—8.1 mass%, respectively. Multifunctional cyclic carbonate systems were also investigated. In the study [45], NIPU foams with different ratio of trifunctional cyclic carbonates were prepared. The decomposition temperature was increased from 270 to 316 °C with the addition of carbonate with higher molar mass. An increase in degradation temperature, when applying cyclic carbonate with higher molar mass, was found by Liu and the co-authors [46]. A variety of amines was used in the reaction with the same type of cyclic carbonate of different molar mass. Temperature of degradation increased with lowering concentration of reactive carbonate groups in carbonates (due to higher molar mass), without regard to the used amines.
In the works of Zareanshahrakia and Asemania et al. [32, 47], two types of cyclic carbonates were applied: a linear bifunctional and a branched trifunctional one. Thermogravimetric study shows that NIPU based on linear cyclic carbonate exhibits higher thermal stability then the branched one. It was postulated that such an effect is due to higher concentration of urethane linkages formed by trifunctional carbonate.
The presence of aromatic moieties in conventional polyurethanes improves their thermal stability [48], following the well-known phenomena that high resonance energy characteristic for aromatic moieties enhances thermal stability of polymers [49, 50]. Such influence was observed also for NIPUs in a work of Karami and co-authors [51]. The main degradation peak for NIPU based on aromatic carbonate was located in higher temperature region, in comparison with the non-aromatic component.
Amine components
Amine components, due to their availability and diversity, are the ones that in NIPU synthesis are the most variable factor. Along this line of interest, amines’ structures and their influence on the thermal stability of NIPU were studied by Malik and Kaur [52]. In this work, four different amines were reacted with canola oil-based cyclic carbonate: 1,2‐ethanediamine (EDA), 1,6‐hexanediamine (HMDA), isophoronediamine (IPDA), and paraphenylenediamine (PPDA) with the molar ratio 1:1—Fig. 4. Thermogravimetric investigations revealed the highest thermal stability for NIPU based on linear shortest amine, and the lowest for cycloaliphatic one. The presented results stand in the contradiction with abovementioned hypothesis that higher amount of urethane bonds (for EDA), decreases the thermal stability. This indicates that, assuming that the degree of conversion for different amines is the same, there are more factors than the concentration of urethane bonds that trigger thermal degradation. Moreover, adding aromatic rings to the structure of amine resulted in higher thermal stability but yet decreased in comparison with EDA-based NIPU what was not observed for aromatic cyclic carbonates.
TGA curves of NIPU based on different amines. Reprinted with permission from Wiley [52]
The influence of amine chain length on the onset degradation temperature was discussed by Zhang a coworkers [34]. Authors observed that with an increase in ratio of amines with long PTMO (polytetramethylene oxide) chains, the temperature at which decomposition starts also increases. It was stated that main factor responsible for such observation is lower concentration of urethane bonds when longer amines are applied. One can conclude that analyzing amine component chain length for short amines, the highest thermal stability exhibits the shortest one, and comparing long chained structures, the one with bigger molecular mass leads to higher onset degradation temperatures. The above conclusions can be confirmed to some extent by the research results obtained by Duval et al. [53]. Different diamines with the number of methylene groups from 4 to 10 were applied, and temperature at which 5% mass loss is a detected decrease when diamine length increases up to 6 methylene moieties in the chain. However, when diamine with 10 methylene group is used, the temperature rises significantly from 233 to 245 °C. The impact of chain structure for long diamine component was also studied [54]. Two types of diamines were used: PTMO-based amine (Jeffamine™ ER-148) and composed only of methylene moieties (Priamine™ 1073) to form NIPU foams. Authors presented TGA results at 30% mass loss during pyrolysis, showing that polyether-based amine display lower decomposition temperature. One has to note that in this work no information about onset degradation temperature is provided, therefore only stability of soft segments is given.
Ratio of cyclocarbonates and amine component also plays an important role in the thermal stability of NIPU. In the work by Wang et al. [55], cyclocarbonates were reacted with isophorone diamine with different molar ratio of components. It was found that formulation in which molar ratio between carbonate and amine components is even (1:1) gives NIPU with the highest thermal stability. Similar study was presented in the work based on linear cyclic carbonates [55]. Here authors used cyclocarbonate to diamine molar ratio from 10:0 to 10:6, indicating highest temperature of 5% mass loss for the biggest concentration of isophorone diamine.
In the work of Catala et al. [56], the impact of crosslinking density in NIPU on its thermal stability was discussed. By using blocking method and a variety of amines, authors obtained NIPUs with different amount of crosslinking points in the matrices. As a result, NIPU with the highest crosslinking density was thermally the most stable, which was explained by creation of a strong, interconnected network that is less prone to decomposition during pyrolysis. Higher thermal stability for crosslinked NIPUs was also reported by Farhadian and coworkers [28]. Three bio-based amines with different amount of amine groups were synthesized and then reacted with identical cyclocarbonate. The highest temperature of 5% mass loss was found for NIPU based on multifunctional amines. As an explanation for such result, authors claimed that both high amount of crosslinks and hydrogen bonds make more thermally resistant NIPUs. Additionally, the improvement of thermal stability by the addition of multifunctional, branched amines, namely diethylenetriamine (DETA) and triethylenetetramine (TETA) was reported in the work of Ke et al. [57].
Pyrolysis and thermo-oxidative decomposition
The conditions of thermogravimetric measurements have a significant impact on the temperatures of the beginning of degradation, its further course, including the number of stages. During pyrolysis process, inert gas flow protects the investigated sample from oxidation, especially at first stages of decomposition. In the case of conventional polyurethanes, usually the onset pyrolysis temperature is higher than during oxidation, and decomposition proceeds in several stages [5, 58, 59]. Interestingly, it for NIPUs different degradation course has been observed in comparison with classical PUs. Carré et al. [60] investigated NIPUs synthesized from sebacic biscyclocarbonates and dimer amines at various ratios. The obtained polymers exhibited typical for NIPUs thermal stability with the onset temperature at 200 °C. Notably, authors found no difference in thermal degradation of NIPUs conducted in inert and oxidative atmosphere. To the same conclusions came authors of the study [31]—thermoxidative measurements show negligible difference of temperatures corresponding to 5 and 30% mass loss compared to studies conducted in an inert gas atmosphere—Fig. 5. Having in mind the difference between PU and NIPU in the presence of additional oxygen in vicinity of urethanes, one can hypothesize that during pyrolysis, at first stage of decomposition, local oxidation can take place. Hence, there may be no distinct difference between the two mechanisms of degradation. In the opposition to that hypothesis, there are the results presented in the different work by Carré and co-authors [61]. The synthesized NIPU exhibit lower onset temperature in the synthetic air than in the nitrogen atmosphere. The gap of ca. 50 °C was explained by an additional oxidation processes occurring in oxygen-containing atmosphere. However, in order to clearly determine the impact of thermal degradation conditions on the course of NIPU decomposition, a more detailed analysis is still required.
Degradation temperatures of PU and PHU materials. Reprinted with permission from Elsevier [31]
Additives
From the application point of view, polymeric matrices often require to be modified in order to enhance their properties. Certainly, the most straightforward method of such modification is addition of inert of reactive filler with different chemical nature, structure and size [62,63,64]. As a field of NIPU composites is relatively new, not much attention was paid to polymer modifications toward higher thermal stability [65]. Here, we discuss some of the most recent works on the influence of additives on thermal stability of NIPUs.
Bio-based additives
Most of the investigations about NIPU synthesis concerns use of bio-based components in order to develop green production paths [23, 66, 67]. It is therefore not surprising that further modification routes involve bio-based additives. Thermal stabilization effects were mostly investigated for lignin and cellulose fillers. One of the most important advantages of lignin and cellulose-based additives is their renewability. Moreover, lignin and cellulose might be obtained from by-products such as wooden chips [68]. However, as is the case for most of bio-derived materials, lignin does not possess strictly defined structure. It is in general a polyphenolic amorphous material, in which botanical origin dictates the proportions of different phenyl-derived units in the lignin [68, 69]. Lignin is used in polymeric materials as reinforcing agent, filler, compatibilizer or stabilizing agent [70]. Cellulose is a linear polysaccharide with backbone consisting of β-1,4 linked glucopyranose rings. It is usually used in form of fibers to reinforce polymer matrices. Due to rather polar and hydrophilic nature cellulose fibers are incompatible with non-polar polymer matrices, and therefore, their use in non-modified form is usually restricted to polar polymer matrices. In case of NIPUs which exhibit polar nature due to the presence of hydroxyurethane moieties, polar nature of the cellulose is an advantage. However, introduction of bio-derived additives into polymer matrix often limits the processing temperatures. If the used additive is polar, such as cellulose, the introduction might also increase hygroscopicity of the composite leading to increased humidity absorption which might be undesirable from the technological point of view [71, 72]. Both lignin and cellulose can be used as fillers in their original form or after chemical or physical modifications.
Zhao et al. [73] studied linear NIPU matrix that was chemically modified by lignin. The lignin acted as a crosslinking agent in the composites/copolymers. Thermal stability of the pristine matrix and lignin-modified NIPUs was studied in inert and oxidative atmospheres. Since, as shown by the authors, the lignin possesses lower thermal stability than the NIPU matrix; the decrease in the thermal stability of composites was observed, with exception of 10 mass% composite (in N2).
The increase was correlated with aromatic moieties that are present in the lignin structure, however, if the aromaticity was the sole factor driving this change, the increase in thermal stability should also be observed for other composites with higher lignin content. Therefore, the lignin influence of other nature should be considered, mainly one associated with crosslinking of the NIPU/lignin material. Crosslinking in general increases the thermal stability of the polymers since cured networks require, apart from main chain decomposition, scission of crosslinking bonds for their molecular mass to be reduced [74]. Therefore, an increase in thermal stability, observed for 10 mass% composite in inert atmosphere, could origin from increased crosslinking degree. For composites with higher lignin content, when the decrease in thermal stability in comparison with the matrix is observed, most probably the effect of crosslinking is overcome with influence of the factor that is low thermal stability of the lignin itself. Zhan et al. [75] prepared NIPU-lignin copolymers by reacting amine-terminated NIPU prepolymer with lignin bearing epoxide rings. The change in the thermal stability of NIPU with lignin introduction might originate not only from incorporating aromatic rings, but also from increasing crosslinking density as well as exchanging hydroxyurethane moieties for secondary amines that exhibit higher thermal stability and reduce oxygen atom content in polymer.
NIPU blends with starch were prepared by Ghasemlou and coworkers [76]; the content of NIPU in blends ranged from 10 to 50 mass%. Thermal stability of the materials was studied under inert atmosphere (N2). Although no comparison to the pure NIPU matrix was made, it was shown that adding NIPUs to the starch film retards the thermal stability by decreasing Tonset temperatures. Thus, the more starch was in the blend, the better was thermal stability. Despite no direct study, it might be therefore suggested that starch can act as a thermal enhancing agent in NIPUs. It is noteworthy that starch is physically blended in the NIPU matrix by utilizing hydrogen bonding between starch OH groups and hydroxyurethene groups present in the NIPU structure.
Nanoadditives
Since the first attempt of using nanomaterials to reinforce polymeric matrices, a vast number of results has been published resulting in better understanding of the effects of nanomaterials on polymers’ behavior and properties [77, 78]. The use of nano-scale modifiers in order to enhance thermal stability is a complex issue, leading sometimes to contradictory conclusions [62, 79]; however, it is also a subject of interest for NIPU composites.
Carbon-based nanoparticles
To the group of carbon-based nanomaterials belong: carbon nanotubes (single or multiwalled) (CNT), graphene oxide (GO) and its derivatives, and carbon quantum dots (QD) [80]. The effect of carbon-based nanoadditives on the thermal stability of various polymers has been broadly discussed [81,82,83]. It was shown that interfacial compatibility between the polymer and the carbon nanofillers is crucial in obtaining composites with enhanced thermal stability [84]. Since NIPUs usually exhibit polar nature due to the presence of hydroxyurethane moieties [85], a good compatibility is expected for nanoadditives of polar nature or for carbon nanostructures equipped with reactive groups that would allow chemical incorporation of the nanoadditive into the polymer chain.
Therefore, one of the promising routes is surface modification of carbon nanoparticles to achieve better compatibility with the NIPU matrix, which in turn should result in mechanical and thermal properties enhancements. The modification of nanostructured carbon materials allows to overcome their hydrophobicity and general chemical inertness that may lead to agglomeration upon introduction to the polymer matrix. Although the need of the modification might be perceived as the unfavorable due to the introduction of an additional necessary step during the preparation of materials, it may lead to compatible nanoadditives of desired thermal properties and dimensional stability maintained even at high temperatures [86].
Modified multiwalled carbon nanotubes/NIPU composites were prepared and examined by He et al. [87]. In order to enhance compatibility with non-isocyanate matrix, MWCNT was functionalized by carboxyl and amine groups. TGA study showed for composites with MWCNT functionalized with COOH groups an increase in the thermal stability, evidenced by higher temperature of 5% mass loss. An opposite effect for the amino group modification was explained by higher water absorption by the system, causing 5% mass loss temperature drop—Fig. 6.
TGA curves of NIPU/MWCNT composites. Reprinted with permission from RSC [87]
Doley et al. [88] studied oil-based non-isocyanate polyurethanes modified with amine-functionalized multiwalled carbon nanotube (AF-CNT) (0.5–2%) that were chemically introduced into the network. The influence of AF-CNT on the thermal stability was studied under nitrogen atmosphere. AF-CNT was shown to increase the thermal stability of the hybrid monotonously up to 1.5 mass%. Upon further increase in filler content, the thermal stability got reduced (still higher than for the matrix, but lower than for 1 mass% composite). T5% was increased by 35 °C upon introduction of 1.5 mass% to the NIPU matrix. An increase in the thermal stability induced by AF-CNT was attributed to the nano-mechanical interlocking of AF-CNTs with the polymer matrix via the polar functionalities that can facilitate electrostatic interactions and to the presence of aromatic moieties in MWCNT.
Doley et al. [89] investigated the effect of amine functionalization of graphene oxide (AF-GO) on the properties of NIPUs. Sunflower oil-based cyclic carbonate was mixed with epoxy resin in a presence of isophorone diamine as a curing agent. Thermal stability investigation of the obtained materials showed an increase in initial degradation temperature from 218 to 312 °C for the highest load of AF-GO (1 mass%); such an improvement was explained by interfacial interactions between highly functionalized GO particles and NIPU matrix.
Although the above works suggest that low loading of carbon nanoadditives functionalized with reactive groups enhance the thermal stability, one need to consider that non-isocyanate polyurethanes are a broad group of polymers with different chemical compositions and chain architectures. Therefore, it needs to be taken into account that the present state of art regarding carbon nanofillers influence on the thermal stability of NIPUs does not allow for drawing unequivocal conclusions. The above is particularly true if we take into consideration that the given nanoadditive can influence the thermal properties differently in non-isocyanate polyurethanes of different chemical composition or chain structure.
Another factor that should be considered is safety. Carbon nanotubes usage rises significant concerns due to their toxicity, since the pulmonary exposure to CNTs may lead to lung inflammation which is usually correlated with development of lung fibrosis and cancer [90].
Silica-based nanoparticles
Nanoparticles based on silica (SNPs) exhibit many advantageous features, such as tailorability of the physicochemical properties, non-toxicity and biocompatibility, and high specific area and thermal insulation, and therefore, SNPs play a growing role in pharmacy and medicine, e.g., as a nanocarriers in drug delivery systems [91,92,93,94,95]. In classical polymer and rubber technology, these nanoparticles were originally introduced to enhance mechanical and thermal properties of composite materials [96,97,98]. In polymer nanocomposites, one of the most widely used silica-based nanoadditives are polyhedral oligomeric silsesquioxane (POSS) nanoparticles that have been applied as three-dimensional nanostructured additives [99,100,101,102,103]. POSS are hybrid type nanostructures of 1–3 nm size with silica cage as a core with organic substituents attached to it. Silica core of POSS exhibits high dimensional stability, while a wide range of possibilities for selecting organic substituents allows for tailoring physical and chemical properties of POSS. As a result, POSS nanoparticles are considered as one of the most favorable nano-reinforcements for high-performance advanced hybrid composites [104].
Silica nanoparticles used in polymer and rubber technology might be used as a self-standing filler, are grafted on a substrate or are combined with other components to form hybrid nanoparticles of more complex structure, such as core–shell nanoparticles.
SiO2 nanoparticles (NP) at high filler loading (20%, 30% and 40%) were applied by Noriega et al. to modify NIPUs [105]. Thermogravimetric curves were recorded in inert atmosphere (N2), and it was revealed that the composite materials exhibit degradation steps at temperatures lower than in pristine matrix.
Moreover, the DTG curves indicate that in the composites, additional degradation steps occur at temperatures lower than the degradation onset for the matrix.
In another work, Hoşgör et al. [106] modified phosphine oxide-based NIPU with spherical silica chemically introduced into the polymer structure via cyclic carbonate groups. Spherical silica did not seem to have any significant effect on the composites first degradation step; however, it shifted the second and third degradation steps toward higher temperatures.
NIPUs with zirconia coated silica core–shell nanoparticles (ZrO2-SiO2 NPs) (0.5–3.0 mass%) were obtained by Farid et al. [107], and the thermal stability was studied in inert atmosphere (N2). However, the first stage of mass loss in the composites begins at much lower temperatures as it does in the non-modified matrix.
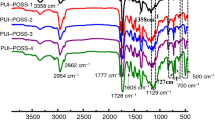
Liu et al. [108] described NIPU modification using chemically linked epoxy-functionalized POSS in the amount of 1–6%. The determined values of Tonset indicate a non-monotonous increase in the thermal stability upon POSS introduction; however, TGA curves show that for composites the gradual mass loss starts at temperatures significantly lower than that for the matrix. Chemical introduction of epoxy-functionalized POSS promotes additional degradation step at 450 °C, which might origin from partial degradation of modified POSS [35]—Fig. 7.
Possible pathway of POSS decomposition. Reprinted with permission from Elsevier [35]
In another work, modification of NIPUs by chemically incorporating into polymer network POSS bearing tri(cyclic carbonate) groups was presented [109]. POSS was introduced in the amount of 5–20 mass%, and the thermal stability of the materials was studied in oxidative atmosphere (air). The POSS incorporation did not affect thermal stability (all materials exhibit the same Tonset temperatures) nor did it influence the decomposition mechanism. However, the impact of POSS was observed during second step of degradation which was shifted from 500 °C up to 550 °C. It was proposed that the partial replacement of PHU segments with POSS cages impeded the release of gaseous products generated during the thermal decomposition process. Chemical modification of NIPU was also performed by MacInnis et al. [110] who introduced monofunctionalized amino-POSS (25 mass%) into the polymer backbone as a chain-terminal group. The thermal stability of the materials was studied under inert atmosphere (N2). The thermogravimetric curves show notable increase in Tonset for composite materials. The increase in the thermal stability was attributed to the presence of POSS. However, it should be noted that composites exhibit 1.5–2.0 times greater average molar mass in comparison with the NIPU oligomer, so the increase in the onset temperature of decomposition could be also attributed to molar mass changes [74]. Interestingly, subsequent introduction of non-chemically bonded POSS caused a further increase in the thermal stability of the hybrids.
As shown in our work [111], physical introduction of POSS into the NIPU matrix allows for enhancing thermal stability in both inert and oxidative atmospheres, and it is much more effective in enhancing thermal properties than chemical incorporation. It is noteworthy that the increase in thermal stability occurred in such composites despite POSS having lower temperature of degradation than the NIPU matrix. The above was attributed to the mutual shielding of POSS and NIPU matrix.
The fact that POSS introduction more readily enhances thermal stability of NIPUs upon physical introduction rather than by chemical incorporating might be associated with the fact that physically added POSS possess some mobility in the network. Upon increasing the temperature, POSS moieties are able to migrate to the surface and form a siliceous protective barrier.
Although silica-based nanoparticles exhibit numerous advantages, as is usually the case with nanoparticles, SNPs also raise some concern regarding health safety. Mass production or broad application may lead to increased risk of human exposure to the nanoparticles that are not immobilized in the bulk of the polymer. Research shows that uncontrollable release of SNPs to the environment might cause adverse cardiovascular pathologies and lead to neurotoxicity in living organisms [112,113,114], turning NPs used as potential drug carriers into health hazardous ones. Therefore, the understanding of life-cycle of SNPs, including their behavior during the thermal degradation of polymer composites, is crucial for ensuring safety of users.
Thermal degradation kinetics
Analysis of thermal degradation kinetics provides valuable information about mechanism that drives thermal decomposition of polymers. During the last decades, conventional polyurethanes have been studied in order to determine kinetics of degradation process, in a vast number of works [115,116,117].
For the NIPU materials, kinetics of degradation process has not been extensively studied. Szwindowska et al. [118] determined energy activation of NIPUs and their components based on thermogravimetric measurement at three different heating rates of 5, 10 and 20 K min−1. By using a model-free Ozawa, Flynn and Wall method authors concluded that bio-based components can provide better thermal properties due to higher activation energy than their petrochemical equivalent. Moreover, in comparison with conventional polyurethanes, the obtained NIPUs exhibited higher activation energy in the first stage of decompositions, allowing to draw conclusions that more energy needs to be deliver in order to cause dissociation of urethane linkage in non-isocyanate polyurethanes.
Conclusions and future outlooks
Based on the literature results and our own research works one can conclude that thermal degradation and stability of NIPUs depend on numerous factors that can act simultaneously. The results obtained showed the influence of ratio and molar mass of carbonate and amine components, and the chemical structure resulting therefrom, on the thermal degradation onset temperature and next decomposition steps. Higher concentration of urethane bonds and the presence of hydroxyl group in the vicinity of urethane groups in NIPUs (compared to PUs) lead, generally, to lower thermal stability. In order to increase the onset temperature of the degradation, branched and/or higher molar mass substrates should be used in the synthesis process. When using various amine components, the main factor influencing thermal stability is the length of their chain. Application of bio- or nano-additives to the NIPU matrices can result in mixed changes in the degradation temperatures, and it shall be studied individually for each composite material.
Despite numerous results on the thermal properties of different NIPUs, some questions still remain unanswered. It would be beneficial to understand how hydroxyl groups in polyhydroxyurethanes influence not only the initial thermal stability, but also next degradation steps under both inert and oxidative atmosphere. In order to draw clear conclusions, individual studies would require standardization of conditions so that it would be possible to determine the impact of selected variables on the thermal stability. In light of the above, it is also important to determine how the conditions of the degradation process (pyrolysis and thermooxidation) affect the degradation course. This is especially true for pyrolysis process that requires a complex temperature protocol. Thus, understanding the influence of thermal analysis conditions on the formation of NIPUs thermal degradation products could lead to the possibility of tailoring the formed products during temperature-assisted decomposition. Such knowledge, along with kinetic analysis of the degradation process, could allow to choose the decomposition pathways that are the safest for the environment and human health in, e.g., thermal recycling installations.
Applying bio- or nanoadditives to the NIPU matrices usually results in changes in the temperatures of degradation which extent depends on the used additive and the mode of their incorporation into the matrix—physical or chemical. As some fillers deteriorate the thermal stability of NIPU matrices, proper selection of an additive is of key importance to obtain NIPU composites or hybrids with enhanced thermal performance. Among additives studied, only a few were found to increase the thermal stability of NIPUs, mainly carbon nanotubes and some types of POSS.
Therefore, achieving full understanding of how the given additive influences NIPUs thermal properties, requires more research. Future works on this topic should focus on conducting comparative studies that would allow explaining the impact of the polymer structure and additives on the thermal degradation behavior. Since NIPU materials usually possess good thermal stability on their own, it would be interesting, from the fundamental and application point of view, to study the influence of cost-effective fillers, such as inexpensive mineral fillers, low-cost carbon-based additives derived from recycled materials, biowastes on the NIPUs thermal stability to fabricate low-cost NIPU-based materials with good thermal properties.
References
Verma D, Okhawilai M, Dalapati GK, Ramakrishna S, Sharma A, Sonar P, et al. Blockchain technology and AI-facilitated polymers recycling: utilization, realities, and sustainability. Polym Compos. 2022;43:8587–601. https://doi.org/10.1002/PC.27054.
AL-Oqla FM, Hayajneh MT, Al-Shrida MM. Mechanical performance, thermal stability and morphological analysis of date palm fiber reinforced polypropylene composites toward functional bio-products. Cellulose. 2022;29:3293–309. https://doi.org/10.1007/S10570-022-04498-6.
Hayeemasae N, Ismail H. Enhancing the thermal stability of natural rubber/recycled ethylene propylene diene rubber blends through the use of bio-compatibilizers. J Vinyl Addit Technol. 2019;25:E155–65. https://doi.org/10.1002/VNL.21674.
Mascarenhas NO, Pereira MA, Pires CAM, Simonelli G, Santos LCL. Production, optimization, and evaluation of thermal stability of palm oil biodiesel produced using a natural coconut oil–based surfactant. Biomass Convers Biorefinery. 2022;1:1–18. https://doi.org/10.1007/S13399-022-03102-Y.
He JJ, Jiang L, Sun JH, Lo S. Thermal degradation study of pure rigid polyurethane in oxidative and non-oxidative atmospheres. J Anal Appl Pyrolysis. 2016;120:269–83. https://doi.org/10.1016/j.jaap.2016.05.015.
Gu X, Mather PT. Entanglement-based shape memory polyurethanes: synthesis and characterization. Polymer. 2012;53:5924–34. https://doi.org/10.1016/J.POLYMER.2012.09.056.
Kausar A, Zulfiqar S, Sarwar MI. High performance segmented polyurethanes derived from a new aromatic diisocyanate and polyol. Polym Degrad Stab. 2013;98:368–76. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2012.09.004.
Liu N, Zhao Y, Kang M, Wang J, Wang X, Feng Y, et al. The effects of the molecular weight and structure of polycarbonatediols on the properties of waterborne polyurethanes. Prog Org Coatings. 2015;82:46–56. https://doi.org/10.1016/J.PORGCOAT.2015.01.015.
Rychlý J, Lattuati-Derieux A, Lavédrine B, Matisová-Rychlá L, Malíková M, Csomorová K, et al. Assessing the progress of degradation in polyurethanes by chemiluminescence and thermal analysis. II Flexible polyether- and polyester-type polyurethane foams. Polym Degrad Stab. 2011;96:462–9. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2011.01.012.
Xie F, Zhang T, Bryant P, Kurusingal V, Colwell JM, Laycock B. Degradation and stabilization of polyurethane elastomers. Prog Polym Sci. 2019;90:211–68. https://doi.org/10.1016/j.progpolymsci.2018.12.003.
Lewicki JP, Pielichowski K, De La Croix PT, Janowski B, Todd D, Liggat JJ. Thermal degradation studies of polyurethane/POSS nanohybrid elastomers. Polym Degrad Stab. 2010;95:1099–105. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2010.02.021.
Fortman DJ, Sheppard DT, Dichtel WR. Reprocessing cross-linked polyurethanes by catalyzing carbamate exchange. Macromolecules. 2019;52:6330–5. https://doi.org/10.1021/ACS.MACROMOL.9B01134.
Oenema J, Liu H, De CN, Eschenbacher A, Van de Vijver R, Weng J, et al. Review on the pyrolysis products and thermal decomposition mechanisms of polyurethanes. J Anal Appl Pyrolysis. 2022;168: 105723. https://doi.org/10.1016/J.JAAP.2022.105723.
Quagliano Amado JC. Thermal resistance properties of polyurethanes and its composites: a short review. J Res Updat Polym Sci. 2019;8:66–84. https://doi.org/10.6000/1929-5995.2019.08.10.
Pielichowski K, Njuguna J, Majka TM. Thermal Degradation of Polymeric Materials. 2nd ed. Elsevier; 2022.
Yang WP, Macosko CW, Wellinghoff ST. Thermal degradation of urethanes based on 4,4′-diphenylmethane diisocyanate and 1,4-butanediol (MDI/BDO). Polymer. 1986;27:1235–40. https://doi.org/10.1016/0032-3861(86)90012-1.
Naik AD, Fontaine G, Bellayer S, Bourbigot S. Salen based Schiff bases to flame retard thermoplastic polyurethane mimicking operational strategies of thermosetting resin. RSC Adv. 2015;5:48224–35. https://doi.org/10.1039/C5RA06242J.
Wołosz D, Parzuchowski PG, Świderska A. Synthesis and characterization of the non-isocyanate poly(carbonate-urethane)s obtained via polycondensation route. Eur Polym J. 2021. https://doi.org/10.1016/j.eurpolymj.2021.110574.
Unverferth M, Kreye O, Prohammer A, Meier MAR. Renewable non-isocyanate based thermoplastic polyurethanes via polycondensation of dimethyl carbamate monomers with diols. Macromol Rapid Commun. 2013;34:1569–74. https://doi.org/10.1002/marc.201300503.
Rokicki G, Parzuchowski PG, Mazurek M. Non-isocyanate polyurethanes: synthesis, properties, and applications. Polym Adv Technol. 2015;26:707–61. https://doi.org/10.1002/pat.3522.
Blattmann H, Fleischer M, Bähr M, Mülhaupt R, Blattmann H, Fleischer M, et al. Isocyanate- and phosgene-free routes to polyfunctional cyclic carbonates and green polyurethanes by fixation of carbon dioxide. Macromol Rapid Commun. 2014;35:1238–54. https://doi.org/10.1002/MARC.201400209.
Filippi L, Meier MAR, Filippi L, Meier RMA. Fully renewable non-isocyanate polyurethanes via the lossen rearrangement. Macromol Rapid Commun. 2021;42:2000440. https://doi.org/10.1002/MARC.202000440.
Ghasemlou M, Daver F, Ivanova EP, Adhikari B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: a review. Eur Polym J. 2019. https://doi.org/10.1016/j.eurpolymj.2019.06.032.
Choong PS, Chong NX, Wai Tam EK, Seayad AM, Seayad J, Jana S. Biobased nonisocyanate polyurethanes as recyclable and intrinsic self-healing coating with triple healing sites. ACS Macro Lett. 2021;10:635–41. https://doi.org/10.1021/ACSMACROLETT.1C00163.
Schmidt S, Ritter BS, Kratzert D, Bruchmann B, Mülhaupt R. Isocyanate-free route to poly(carbohydrate-urethane) thermosets and 100% bio-based coatings derived from glycerol feedstock. Macromolecules. 2016;49:7268–76. https://doi.org/10.1021/ACS.MACROMOL.6B01485.
Mhd Haniffa MAC, Munawar K, Ching YC, Illias HA, Chuah CH. Bio-based poly(hydroxy urethane)s synthesis and pre/post-functionalization. Chem—An Asian J. 2021;16:1281–97. https://doi.org/10.1002/ASIA.202100226.
Poussard L, Mariage J, Grignard B, Detrembleur C, Jéroîme C, Calberg C, et al. Non-isocyanate polyurethanes from carbonated soybean oil using monomeric or oligomeric diamines to achieve thermosets or thermoplastics. Macromolecules. 2016;49:2162–71. https://doi.org/10.1021/acs.macromol.5b02467.
Farhadian A, Ahmadi A, Omrani I, Miyardan AB, Varfolomeev MA, Nabid MR. Synthesis of fully bio-based and solvent free non-isocyanate poly (ester amide/urethane) networks with improved thermal stability on the basis of vegetable oils. Polym Degrad Stab. 2018;155:111–21. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2018.07.010.
Cornille A, Guillet C, Benyahya S, Negrell C, Boutevin B, Caillol S. Room temperature flexible isocyanate-free polyurethane foams. Eur Polym J. 2016;84:873–88. https://doi.org/10.1016/j.eurpolymj.2016.05.032.
Błażek K, Kasprzyk P, Datta J. Diamine derivatives of dimerized fatty acids and bio-based polyether polyol as sustainable platforms for the synthesis of non-isocyanate polyurethanes. Polymer. 2020;205: 122768. https://doi.org/10.1016/J.POLYMER.2020.122768.
Cornille A, Michaud G, Simon F, Fouquay S, Auvergne R, Boutevin B, et al. Promising mechanical and adhesive properties of isocyanate-free poly(hydroxyurethane). Eur Polym J. 2016;84:404–20. https://doi.org/10.1016/J.EURPOLYMJ.2016.09.048.
Asemani H, Zareanshahraki F, Mannari V. Design of hybrid nonisocyanate polyurethane coatings for advanced ambient temperature curing applications. J Appl Polym Sci. 2019;136:47266. https://doi.org/10.1002/app.47266.
Matsumoto K, Kokai A, Endo T. Synthesis and properties of novel poly(hydroxyurethane) from difunctional alicyclic carbonate and m-xylylenediamine and its possibility as gas barrier materials. Polym Bull. 2016;73:677–86. https://doi.org/10.1007/s00289-015-1513-2.
Zhang K, Nelson AM, Talley SJ, Chen M, Margaretta E, Hudson AG, et al. Non-isocyanate poly(amide-hydroxyurethane)s from sustainable resources. Green Chem. 2016;18:4667–81. https://doi.org/10.1039/C6GC01096B.
Bukowczan A, Stachak P, Łukaszewska I, Majka TM, Hebda E, Pielichowski K. Pyrolysis and thermal degradation studies of non-isocyanate polyurethanes modified by polyhedral oligomeric silsesquioxanes. Thermochim Acta. 2023;723: 179484. https://doi.org/10.1016/J.TCA.2023.179484.
Monie F, Grignard B, Thomassin J, Mereau R, Tassaing T, Jerome C, et al. Chemo- and regioselective additions of nucleophiles to cyclic carbonates for the preparation of self-blowing non-isocyanate polyurethane foams. Angew Chemie. 2020;132:17181–9. https://doi.org/10.1002/ange.202006267.
Duval C, Kébir N, Charvet A, Martin A, Burel F. Synthesis and properties of renewable nonisocyanate polyurethanes (NIPUs) from dimethylcarbonate. J Polym Sci Part A Polym Chem. 2015;53:1351–9. https://doi.org/10.1002/pola.27568.
Lee CF, Chen CW, Rwei SP, Chuang FS. Thermal behavior and morphology of thermoplastic polyurethane derived from different chain extenders of 1,3-and 1,4-butanediol. Appl Sci. 2021;11:1–18. https://doi.org/10.3390/app11020698.
Ornaghi HL, Nohales A, Asensio M, Gómez CM, Bianchi O. Effect of chain extenders on the thermal and thermodegradation behavior of carbonatodiol thermoplastic polyurethane. Polym Bull. 2023. https://doi.org/10.1007/s00289-023-04812-7.
Favero D, Marcon V, Figueroa CA, Gómez CM, Cros A, Garro N, et al. Effect of chain extender on the morphology, thermal, viscoelastic, and dielectric behavior of soybean polyurethane. J Appl Polym Sci. 2021;138:1–16. https://doi.org/10.1002/app.50709.
Wang TL, Hsieh TH. Effect of polyol structure and molecular weight on the thermal stability of segmented poly(urethaneureas). Polym Degrad Stab. 1997;55:95–102. https://doi.org/10.1016/S0141-3910(96)00130-9.
Song YM, Chen WC, Yu TL, Linliu K, Tseng YH. Effect of isocyanates on the crystallinity and thermal stability of polyurethanes. J App PolyM Sci. 1996. https://doi.org/10.1002/(SICI)1097-4628(19961031)62:5.
Chattopadhyay DK, Webster DC. Thermal stability and flame retardancy of polyurethanes. Prog Polym Sci. 2009;34:1068–133. https://doi.org/10.1016/J.PROGPOLYMSCI.2009.06.002.
Lefebvre J, Bastin B, Le Bras M, Duquesne S, Paleja R, Delobel R. Thermal stability and fire properties of conventional flexible polyurethane foam formulations. Polym Degrad Stab. 2005;88:28–34. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2004.01.025.
Blattmann H, Lauth M, Mülhaupt R. Flexible and bio-based nonisocyanate polyurethane (NIPU) foams. Macromol Mater Eng. 2016;301:944–52. https://doi.org/10.1002/mame.201600141.
Liu G, Wu G, Huo S, Jin C, Kong Z. Synthesis and properties of non-isocyanate polyurethane coatings derived from cyclic carbonate-functionalized polysiloxanes. Prog Org Coatings. 2017;112:169–75. https://doi.org/10.1016/j.porgcoat.2017.07.013.
Zareanshahraki F, Asemani HR, Skuza J, Mannari V. Synthesis of non-isocyanate polyurethanes and their application in radiation-curable aerospace coatings. Prog Org Coatings. 2020;138: 105394. https://doi.org/10.1016/j.porgcoat.2019.105394.
Rogulska M, Kultys A, Podkościelny W. Studies on thermoplastic polyurethanes based on new diphenylethane-derivative diols. II synthesis and characterization of segmented polyurethanes from HDI and MDI. Eur Polym J. 2007;43:1402–14. https://doi.org/10.1016/J.EURPOLYMJ.2007.01.014.
Fina A, Tabuani D, Carniato F, Frache A, Boccaleri E, Camino G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim Acta. 2006;440:36–42. https://doi.org/10.1016/j.tca.2005.10.006.
Blanco I, Abate L, Bottino FA, Bottino P. Thermal degradation of hepta cyclopentyl, mono phenyl-polyhedral oligomeric silsesquioxane (hcp-POSS)/polystyrene (PS) nanocomposites. Polym Degrad Stab. 2012;97:849–55. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2012.03.041.
Karami Z, Kabiri K, Zohuriaan-Mehr MJ. Non-isocyanate polyurethane thermoset based on a bio-resourced star-shaped epoxy macromonomer in comparison with a cyclocarbonate fossilbased epoxy resin: a preliminary study on thermomechanical and antibacterial properties. J CO Util. 2019. https://doi.org/10.1016/J.JCOU.2019.08.009.
Malik M, Kaur R. Synthesis of NIPU by the carbonation of canola oil using highly efficient 5,10,15-tris(pentafluorophenyl)corrolato-manganese(III) complex as novel catalyst. Polym Adv Technol. 2018;29:1078–85. https://doi.org/10.1002/pat.4219.
Duval C, Kébir N, Jauseau R, Burel F. Organocatalytic synthesis of novel renewable non-isocyanate polyhydroxyurethanes. J Polym Sci Part A Polym Chem. 2016;54:758–64. https://doi.org/10.1002/POLA.27908.
Cornille A, Dworakowska S, Bogdal D, Boutevin B, Caillol S. A new way of creating cellular polyurethane materials: NIPU foams. Eur Polym J. 2015;66:129–38. https://doi.org/10.1016/j.eurpolymj.2015.01.034.
Wang C, Wu Z, Tang L, Qu J. Synthesis and properties of cyclic carbonates and non-isocyanate polyurethanes under atmospheric pressure. Prog Org Coatings. 2019;127:359–65. https://doi.org/10.1016/j.porgcoat.2018.11.040.
Catal J, Guerra I, GarcaVargas JM, Ramos MJ, Garca MT, Rodrguez JF. Tailor-made bio-based non-isocyanate polyurethanes (NIPUs). Polymers. 2023. https://doi.org/10.3390/polym15061589.
Ke J, Li X, Wang F, Jiang S, Kang M, Wang J, et al. Non-isocyanate polyurethane/epoxy hybrid materials with different and controlled architectures prepared from a CO 2-sourced monomer and epoxy via an environmentally-friendly route. RSC Adv. 2017;7:28841–52. https://doi.org/10.1039/c7ra04215a.
Duquesne S, Le Bras M, Bourbigot S, Delobel R, Camino G, Eling B, et al. Thermal degradation of polyurethane and polyurethane/expandable graphite coatings. Polym Degrad Stab. 2001;74:493–9. https://doi.org/10.1016/S0141-3910(01)00177-X.
Shufen L, Zhi J, Kaijun Y, Shuqin Y, Chow WK. Studies on the thermal behavior of polyurethanes. Polym Plast Technol Eng. 2006;45:95–108. https://doi.org/10.1080/03602550500373634.
Carré C, Bonnet L, Avérous L. Original biobased nonisocyanate polyurethanes: solvent- and catalyst-free synthesis, thermal properties and rheological behaviour. RSC Adv. 2014;4:54018–25. https://doi.org/10.1039/c4ra09794g.
Carré C, Zoccheddu H, Delalande S, Pichon P, Avérous L. Synthesis and characterization of advanced biobased thermoplastic nonisocyanate polyurethanes, with controlled aromatic-aliphatic architectures. Eur Polym J. 2016;84:759–69. https://doi.org/10.1016/j.eurpolymj.2016.05.030.
Chrissafis K, Bikiaris D. Can nanoparticles really enhance thermal stability of polymers? Part I: an overview on thermal decomposition of addition polymers. Thermochim Acta. 2011;523:1–24. https://doi.org/10.1016/J.TCA.2011.06.010.
Hsissou R, Seghiri R, Benzekri Z, Hilali M, Rafik M, Elharfi A. Polymer composite materials: a comprehensive review. Compos Struct. 2021;262: 113640. https://doi.org/10.1016/J.COMPSTRUCT.2021.113640.
Ray S, Cooney RP. Thermal Degradation of Polymer and Polymer Composites. In: Handbook of Environmental Degradation of Materials. Third Ed. 2018; 185–206. https://doi.org/10.1016/B978-0-323-52472-8.00009-5
Stachak P, Łukaszewska I, Hebda E, Pielichowski K. Recent advances in fabrication of non-isocyanate polyurethane-based composite materials. Materials. 2021;14:3497. https://doi.org/10.3390/MA14133497.
Ling Z, Zhou Q. Synthesis and properties of linseed oil-based waterborne non-isocyanate polyurethane coating. Green Chem. 2023;25:10082–90. https://doi.org/10.1039/D3GC03249C.
Khatoon H, Iqbal S, Irfan M, Darda A, Rawat NK. A review on the production, properties and applications of non-isocyanate polyurethane: a greener perspective. Prog Org Coatings. 2021;154: 106124. https://doi.org/10.1016/J.PORGCOAT.2020.106124.
Kumar MNS, Mohanty AK, Erickson L, Misra M. Lignin and its applications with polymers. J Biobased Mater Bioenerg. 2009;3:1–24. https://doi.org/10.1166/JBMB.2009.1001.
Boeriu CG, Bravo D, Gosselink RJA, Van Dam JEG. Characterisation of structure-dependent functional properties of lignin with infrared spectroscopy. Ind Crops Prod. 2004;20:205–18. https://doi.org/10.1016/J.INDCROP.2004.04.022.
Polat Y, Stojanovska E, Negawo TA, Doner E, Kilic A. Lignin as an additive for advanced composites. Green Energy Technol. 2017. https://doi.org/10.1007/978-3-319-46610-1_4.
Miao C, Hamad WY. Cellulose reinforced polymer composites and nanocomposites: a critical review. Cellulose. 2013;20:2221–62. https://doi.org/10.1007/S10570-013-0007-3.
Thakur VK, Thakur MK. Processing and characterization of natural cellulose fibers/thermoset polymer composites. Carbohydr Polym. 2014;109:102–17. https://doi.org/10.1016/J.CARBPOL.2014.03.039.
Zhao W, Liang Z, Feng Z, Xue B, Xiong C, Duan C, et al. New kind of lignin/polyhydroxyurethane composite: green synthesis, smart properties, promising applications, and good reprocessability and recyclability. ACS Appl Mater Interfaces. 2021;13:28938–48. https://doi.org/10.1021/ACSAMI.1C06822.
Tomić NZ. (2020) Thermal studies of compatibilized polymer blends. In: Compat Polym Blends Micro Nano Scale Phase Morphol Interphase Charact Prop. 489–510. https://doi.org/10.1016/B978-0-12-816006-0.00017-7
Zhang T, Xue B, Yan Q, Yuan Y, Tan J, Guan Y, et al. New kinds of lignin/non-isocyanate polyurethane hybrid polymers: facile synthesis, smart properties and adhesive applications. Ind Crops Prod. 2023;199: 116706. https://doi.org/10.1016/J.INDCROP.2023.116706.
Ghasemlou M, Daver F, Ivanova EP, Brkljaca R, Adhikari B. Assessment of interfacial interactions between starch and non-isocyanate polyurethanes in their hybrids. Carbohydr Polym. 2020;246: 116656. https://doi.org/10.1016/J.CARBPOL.2020.116656.
Hassan T, Salam A, Khan A, Khan SU, Khanzada H, Wasim M, et al. Functional nanocomposites and their potential applications: a review. J Polym Res. 2021;28:1–22. https://doi.org/10.1007/S10965-021-02408-1.
Hemath M, Mavinkere Rangappa S, Kushvaha V, Dhakal HN, Siengchin S. A comprehensive review on mechanical, electromagnetic radiation shielding, and thermal conductivity of fibers/inorganic fillers reinforced hybrid polymer composites. Polym Compos. 2020;41:3940–65. https://doi.org/10.1002/PC.25703.
Bikiaris D. Can nanoparticles really enhance thermal stability of polymers? Part II: an overview on thermal decomposition of polycondensation polymers. Thermochim Acta. 2011;523:25–45. https://doi.org/10.1016/J.TCA.2011.06.012.
Patel KD, Singh RK, Kim HW. Carbon-based nanomaterials as an emerging platform for theranostics. Mater Horizons. 2019;6:434–69. https://doi.org/10.1039/C8MH00966J.
Azimpour-Shishevan F, Akbulut H, Mohtadi-Bonab MA. Synergetic effects of carbon nanotube and graphene addition on thermo-mechanical properties and vibrational behavior of twill carbon fiber reinforced polymer composites. Polym Test. 2020;90: 106745. https://doi.org/10.1016/J.POLYMERTESTING.2020.106745.
Iqbal A, Saeed A, Ul-Hamid A. A review featuring the fundamentals and advancements of polymer/CNT nanocomposite application in aerospace industry. Polym Bull. 2021;78:539–57. https://doi.org/10.1007/S00289-019-03096-0.
Abubakre OK, Medupin RO, Akintunde IB, Jimoh OT, Abdulkareem AS, Muriana RA, et al. Carbon nanotube-reinforced polymer nanocomposites for sustainable biomedical applications: a review. J Sci Adv Mater Devices. 2023;8: 100557. https://doi.org/10.1016/J.JSAMD.2023.100557.
Zeynalov EB, Agaguseynova MM, Salmanova NI. Effect of nanocarbon additives on stability of polymer composites. Izv Vyss Uchebn Zaved, KhimKhimT. 2020;63:4–12. https://doi.org/10.6060/ivkkt.20206311.6213.
Łukaszewska I, Bukowczan A, Raftopoulos KN, Pielichowski K. Water-polymer interactions and mechanisms of water-driven glass transition decrease in non-isocyanate polyhydroxyurethanes with varying hydration sites. Polymer. 2024;302: 127060. https://doi.org/10.1016/J.POLYMER.2024.127060.
Luo Z, Oki A, Carson L, Adams L, Neelgund G, Soboyejo N, et al. Thermal stability of functionalized carbon nanotubes studied by in situ transmission electron microscopy. Chem Phys Lett. 2011;513:88–93. https://doi.org/10.1016/j.cplett.2011.07.072.
He X, Xu X, Bo G, Yan Y. Studies on the effects of different multiwalled carbon nanotube functionalization techniques on the properties of bio-based hybrid non-isocyanate polyurethane. RSC Adv. 2020;10:2180–90. https://doi.org/10.1039/c9ra08695a.
Doley S, Agarwal V, Bora A, Borah D, Dolui SK. Development of sunflower oil-based nonisocyanate polyurethane/multiwalled carbon nanotube composites with improved physico-chemical and microwave absorption properties. Polym Compos. 2019;40:E1120–30. https://doi.org/10.1002/PC.24897.
Doley S, Sarmah A, Sarkar C, Dolui SK. In situ development of bio-based polyurethane-blend-epoxy hybrid materials and their nanocomposites with modified graphene oxide via non-isocyanate route. Polym Int. 2018;67:1062–9. https://doi.org/10.1002/pi.5612.
Luanpitpong S, Wang L, Rojanasakul Y. The effects of carbon nanotubes on lung and dermal cellular behaviors. Nanomedicine (Lond). 2014;9:895. https://doi.org/10.2217/NNM.14.42.
Yang Y, Zhang M, Song H, Yu C. Silica-based nanoparticles for biomedical applications: from nanocarriers to biomodulators. Acc Chem Res. 2020;53:1545–56. https://doi.org/10.1021/ACS.ACCOUNTS.0C00280.
Murugadoss S, Lison D, Godderis L, Van Den Brule S, Mast J, Brassinne F, et al. (2017) Toxicology of silica nanoparticles an update. Arch Toxicol 91:2967–3010. https://doi.org/10.1007/S00204-017-1993-Y
Yang Y, Yu C. Advances in silica based nanoparticles for targeted cancer therapy. Nanomed Nanotechnol Biol Med. 2016;12:317–32. https://doi.org/10.1016/J.NANO.2015.10.018.
Manzano M, Vallet-Regí M, Manzano M, Vallet-Regí M. Mesoporous silica nanoparticles for drug delivery. Adv Funct Mater. 2020;30:1902634. https://doi.org/10.1002/ADFM.201902634.
Bhatt H, Bahadur J, Checker R, Ajgaonkar P, Vishwakarma SR, Sen D. Influence of molecular interactions on structure, controlled release and cytotoxicity of curcumin encapsulated chitosan—silica nanostructured microspheres. Coll Surf B Biointerfaces. 2021;208: 112067. https://doi.org/10.1016/J.COLSURFB.2021.112067.
Jeziorska R, Szadkowska A, Zielecka M, Wenda M, Kepska B. Morphology and thermal properties of HDPE nanocomposites: effect of spherical silica surface modification and compatibilizer. Polym Degrad Stab. 2017;145:70–8. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2017.06.007.
Guo Q, Zhu P, Li G, Wen J, Wang T, Lu DD, et al. Study on the effects of interfacial interaction on the rheological and thermal performance of silica nanoparticles reinforced epoxy nanocomposites. Compos Part B Eng. 2017;116:388–97. https://doi.org/10.1016/J.COMPOSITESB.2016.10.081.
Serkis M, Špírková M, Kredatusová J, Hodan J, Bureš R. Organic–inorganic nanocomposite films made from polyurethane dispersions and colloidal silica particles. Compos Interfaces. 2016;23:157–73. https://doi.org/10.1080/09276440.2016.1124666.
Majka TM, Raftopoulos KN, Pielichowski K. The influence of POSS nanoparticles on selected thermal properties of polyurethane-based hybrids. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-017-6942-8.
Najaf Oshani B, Davachi SM, Hejazi I, Seyfi J, Khonakdar HA, Abbaspourrad A. Enhanced compatibility of starch with poly(lactic acid) and poly(ɛ-caprolactone) by incorporation of POSS nanoparticles: Study on thermal properties. Int J Biol Macromol. 2019;141:578–84. https://doi.org/10.1016/J.IJBIOMAC.2019.09.026.
Xu Y, Long J, Zhang R, Du Y, Guan S, Wang Y, et al. Greatly improving thermal stability of silicone resins by modification with POSS. Polym Degrad Stab. 2020;174: 109082. https://doi.org/10.1016/J.POLYMDEGRADSTAB.2020.109082.
Mohamed MG, Mansoure TH, Takashi Y, Mohamed Samy M, Chen T, Kuo SW. Ultrastable porous organic/inorganic polymers based on polyhedral oligomeric silsesquioxane (POSS) hybrids exhibiting high performance for thermal property and energy storage. Microporous Mesoporous Mater. 2021;328: 111505. https://doi.org/10.1016/J.MICROMESO.2021.111505.
Bukowczan A, Raftopoulos KN, Czajkowski M, Szefer E, Hebda E, Pielichowski K. Liquid crystalline polyurethanes modified by trisilanolisobutyl-POSS. J Mol Liq. 2022;348:118069. https://doi.org/10.1016/J.MOLLIQ.2021.118069.
Devaraju S, Alagar M. (2021) POSS nanoparticles: Synthesis, characterization, and properties. Polyhedral Oligomeric Silsesquioxane Polym Nanocomposites. https://doi.org/10.1016/B978-0-12-821347-6.00005-6
Noriega NE, Carrillo A, Castillo SJ, Mota ML. Production and characterization of non-isocyanate polyurethane/SiO2 films through a sol-gel process for thermal insulation applications. Polymers. 2019;11:1596–8. https://doi.org/10.3390/POLYM11101596.
Hogör Z, Kayaman-Apohan N, Karata S, Mencelolu Y, Güngör A. Preparation and characterization of phosphine oxide based polyurethane/silica nanocomposite via non-isocyanate route. Prog Org Coatings. 2010;69:366–75. https://doi.org/10.1016/J.PORGCOAT.2010.07.010.
Farid ME, El-Sockary MA, El-Saeed AM, Hashem AI, Abo Elenien OM, Selim MS, et al. An eco-friendly non-isocyanate polyurethane treated by CO2 as flame retardant nanocomposite coating/ZrO2@SiO2. Mater Res Express. 2019;6:065042. https://doi.org/10.1088/2053-1591/AB0DA3.
Liu G, Wu G, Chen J, Huo S, Jin C, Kong Z. Synthesis and properties of POSS-containing gallic acid-based non-isocyanate polyurethanes coatings. Polym Degrad Stab. 2015;121:247–52. https://doi.org/10.1016/j.polymdegradstab.2015.09.013.
Liu W, Hang G, Mei H, Li L, Zheng S. Nanocomposites of polyhydroxyurethane with poss microdomains: synthesis via non-isocyanate approach Morphologies and Reprocessing Properties. Polymers. 2022;14:1331. https://doi.org/10.3390/POLYM14071331.
MacInnis CM, Younes GR, Marić M. The effect of polyhedral oligomeric silsesquioxane fillers in non-isocyanate polyurethane hybrid resins. J Appl Polym Sci. 2022;139: e53225. https://doi.org/10.1002/APP.53225.
Łukaszewska I, Bukowczan A, Raftopoulos KN, Pielichowski K. Spider-like POSS in NIPU webs: enhanced thermal stability and unique swelling behavior. J Polym Res. 2023. https://doi.org/10.1007/s10965-023-03834-z.
Guo C, Liu Y, Li Y. Adverse effects of amorphous silica nanoparticles: focus on human cardiovascular health. J Hazard Mater. 2021. https://doi.org/10.1016/J.JHAZMAT.2020.124626.
Shukur A, Azzawi M, Farooq A, Whitehead D. (2022) Synthesis of silica nanoparticles for biological applications. In: Nanoparticle Ther Prod Technol Types Nanoparticles, Regul Asp https://doi.org/10.1016/B978-0-12-820757-4.00014-4
Zhong C, He M, Lou K, Gao F. The application, neurotoxicity, and related mechanism of silica nanoparticles. Neurotox Nanomater Nanomed. 2017. https://doi.org/10.1016/B978-0-12-804598-5.00010-6.
Agic´ A, Emi A, Govorcˆin G, Bajsic´ B. Strategy for kinetic parameter estimation—thermal degradation of polyurethane elastomers. J Appl Polym Sci. 2007;103:764–72. https://doi.org/10.1002/APP.25040.
Li XB, Cao HB, Zhang Y. Thermal degradation kinetics of rigid polyurethane foams blown with water. J Appl Polym Sci. 2006;102:4149–56. https://doi.org/10.1002/APP.24379.
Bárbara BD, Araújo NRS, Ligório RF, Pujatti FJP, Yoshida MI, Sebastião RCO. Comparative kinetic study of automotive polyurethane degradation in non-isothermal and isothermal conditions using artificial neural network. Thermochim Acta. 2018;666:116–23. https://doi.org/10.1016/J.TCA.2018.06.014.
Parcheta-Szwindowska P, Rohde K, Datta J. Bio-derived polyurethanes obtained by non-isocyanate route using polyol-based bis(cyclic carbonate)s-studies on thermal decomposition behavior. J Therm Anal Calorim. 2022;147:13329–39. https://doi.org/10.1007/s10973-022-11679-9.
Acknowledgements
Polish National Science Center is gratefully acknowledged for financial support under contract No. DEC-2022/45/B/ST8/03060.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bukowczan, A., Łukaszewska, I. & Pielichowski, K. Thermal degradation of non-isocyanate polyurethanes. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13306-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13306-1