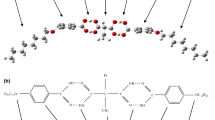
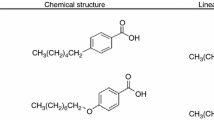
Abstract
Four binary mixtures are isolated from the hydrogen bond liquid crystalline mesogenic complexes comprising methyl malonic acid (MM) and p-n heptyloxy benzoic acid (7BAO) abbreviated as MM + 7BAO & p-n dodecyloxy (12BAO) benzoic acid abbreviated as MM + 12BAO as the precursors exhibit rich phase polymorphism. Variation in the even set of molar proportion in steps of 0.2 leads to the formation of four binary mixtures. Each of the precursors used for the preparation of binary mixtures are treated as X and Y component whose molar ratio varies accordingly. Spectroscopic analysis of the binaries confirms the hydrogen bond existence between the binary mixtures. Phase variance and their textural observations are made through polarizing optical microscopic studies. Thermal properties possessed by these binary mixtures such as phase transition temperatures, enthalpy values, thermal equilibrium, thermal stability, order of phase transition and specific heat capacity are extensively investigated from the differential scanning thermograms obtained for the binaries prepared. These calorimetric investigations suggest the mesophases of the binaries for apt display, thermal and optical applications.








Similar content being viewed by others
References
Carlton RJ, Hunter JT, Miller DS, Abbasi R, Mushenheim PC, Tan LN, et al. Chemical and biological sensing using liquid crystals. Liq Cryst Rev. 2013;1:29–51. https://doi.org/10.1080/21680396.2013.769310.
Kato T, Uchida J, Ichikawa T, Sakamoto T. Functional Liquid crystals towards the next generation of materials. Angew Chem Int Ed. 2018;57:4355–71. https://doi.org/10.1002/anie.201711163.
Kato T, Kato T, Fréchet JMJ, Uryu T, Kaneuchi F, Jin C, et al. Hydrogen-bonded liquid crystals built from hydrogen-bonding donors and acceptors Infrared study on the stability of the hydrogen bond between carboxylic acid and pyridyl moieties. Liq Cryst. 2006;33:1429–37. https://doi.org/10.1080/02678290601119807.
Kato T, Frechet JM, Wilson PG, Saito T, Uryu T, Fujishima A, Jin C, Kaneuchi F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenic bipyridyl compounds through complexation between hydrogen-bond donor and acceptor moieties. Chem Mater. 2002;5:1094–100.
Rohini P, Pongali Sathya Prabu N, Madhu Mohan MLN. Comparison of mesomorphic properties exhibited by linear hydrogen bonded thermotropic liquid crystals. Mol Cryst Liq Cryst. 2016;631:74–91. https://doi.org/10.1080/15421406.2016.1149022.
Smaisim GF, Mohammed KJ, Hadrawi SK, Kianfar E, Köten H. Properties and application of nanostructure in liquid crystals: review. Bionanoscience. 2023;2:819–39.
Pal K, Si A, El-Sayyad GS, Abd Elkodous M, Kumar R, El-Batal AI, Kralj S, Thomas S. Cutting edge development on grapheme derivatives modified by liquid crystal and Cds/TiO2 hybrid matrix: optoelectronics and biotechnological aspects. Crit Rev Solid State Mater Sci. 2020;46:385–449.
Pal K, Aljabali AA, Kralj S, Thomas S, de Souza FG. Graphene-assembly liquid crystalline and nanopolymer hybridization: a review on switchable device implementations. Chemosphere. 2021;263:128104.
Xin H, Chen H, Song P, Sun Q. Alignment control of thermotropic liquid crystals by topography and chemical functionality of a surface: a review. Mater Today Commun. 2023;36:106680.
Pal K, Asthana N, Aljabali AA, Bhardwaj SK, Kralj S, Penkova A, Thomas S, Zaheer T, de Souza FG. A critical review on multifunctional smart materials ‘nanographene’ emerging avenue: nano-imaging and biosensor applications. Crit Rev Solid State Mater Sci. 2022;47:691.
Pal K, Sajjadifar S, Abd Elkodous M, Alli YA, Gomes F, Jeevanandam J, Thomas S, Sigov A. Soft, self-assembly liquid crystalline nanocomposite for superior switching. Electron Mater Lett. 2018;15:84–101.
Hoseini SS, Separdar L, Izadneshan H. Effect of molecular aspect ratio on structure, dynamics and phase stability of thermotropic liquid crystals studied by molecular dynamics simulation. Solid State Commun. 2023;366–367: 115147.
Asiya SI, Kaushik Pal, Introduction of ZnO nanomaterial integration nanospikes to nanocombs dispersed into HBLCs phase transition and novel switching. In Woodhead Publishing Series in Electronic and Optical Materials, Functional Materials Processing for Switchable Device Modulation. Woodhead Publishing, 2022, p. 3–22.
Asiya SI, Kyzas GZ, Pal K, de Souza Jr FG. nanomaterials for industrial-scale applications: systematic review. J Mol Struct. 2021;1239:130518.
Salah MB, Nasri R, Alharbi AN, Althagafi TM, Soltani T. Thermotropic liquid crystal doped with ferroelectric nanoparticles: electrical behavior and ion trapping phenomenon. J Mol Liq. 2021;357:130518.
Wang S, Ma S, Li Na, Jie S, Luo Y, Gao X. Branched thermotropic liquid crystal polymer with favourable processability and dielectric properties. Eur Polym J. 2023;196: 112302.
Pal K, Maiti UN, Majumder TP, Debnath SC, Ghosh S, Roy SK, Otón JM. Switching of ferroelectric liquid crystal doped with cetyltrimethylammonium bromide-assisted CdS nanostructures. J Nanotechnol. 2013;24: 125702.
Wei P, Lou H, Yan J, Li L, Zhang Y, Xia Y, Wang Y, Wang Y. Synthesis and properties of high performance aromatic thermotropic liquid crystal copolyesters based on naphthalene ring structure. Polymer. 2022;240: 124472.
Rajanandkumar R, Pongali Sathya Prabu N, Murugadass K, Madhu Mohan MLN. A study on polymorphism of hydrogen-bonded thermotropic liquid crystals. Phase Transit. 2016;89:928–43. https://doi.org/10.1080/01411594.2015.1114618.
Sangameswari G, Sathya Prabu NP, Madhu Mohan MLN. Binary mixtures of hydrogen-bonded ferroelectric liquid crystals: thermal span enhancement in smectic X* phase. Zeitschrift für Naturforschung A. 2015;70:757–74. https://doi.org/10.1515/zna-2015-0164.
Rohini P, Pongali Sathya Prabu N, Madhu Mohan MLN. Studies on thermotropic hydrogen bonded binary mixtures. Mole Cryst Liq Cryst. 2019;690:23–42. https://doi.org/10.1080/15421406.2019.1680160.
Chandrasekar G, PongaliSathyaPrabu N, Madhu Mohan MLN. Design, synthesis and characterization of hydrogen bonded liquid crystals formed between methyl malonic acid and pn- alkyloxy / alkyl benzoic acids. Mol Cryst Liq Cryst. 2017;652:23–40.
Gray GW, Goodby JW. Smectic liquid crystals: textures and structures. Acta Crystallogr B. 2023;41:205–6.
Kavitha C, Prabu NPS, Madhu Mohan MLN. Thermal analysis of supramolecular hydrogen-bonded liquid crystals formed by nonyloxy and alkyl benzoic acids. Mol Cryst Liq Cryst. 2013;574:96–113. https://doi.org/10.1080/15421406.2012.762500.
Pavia DL, Lampman GM, Kriz GS, Vyvyan JA. Introduction to spectroscopy. Cengage learning; 2014.
Sangameswari G, Pongali Sathya Prabu N, Madhu Mohan MLN. Thermal analysis of hydrogen-bonded ferroelectric liquid crystals. J Therm Anal Calorim. 2017;128:369–86. https://doi.org/10.1007/s10973-016-5925-5.
Kishor MH, Madhu Mohan MLN. Calorimetric study of smectic x* and validation of dynamic memory in ferroelectric binary complexes. J Therm Anal Calorim. 2022;147:3553–66. https://doi.org/10.1007/s10973-021-10797-0.
Efremova EI, Kydryashova ZA, Nosikova LA, Kovshik AP, Dobrun LA, Melnikov AB. Phase diagram and dielectric studies in hydrogen-bonded liquid crystal system. Mol Cryst Liq Cryst. 2016;626:12–20.
Okumuş M, Dindar S. Thermal and mesomorphic properties of 8OBA/nABA(n = 3, 4) hydrogen-bonded liquid crystalline complexes. J Therm Anal Calorim. 2023;148:11577–87.
Sathya Prabu NP, Madhu Mohan MLN. Thermal analysis of hydrogen bonded benzoic acid liquid crystals. J Therm Anal Calorim. 2013;113:811–20. https://doi.org/10.1007/s10973-012-2812-6.
Chandrasekar G, Prabu NPS, Mohan MLNM. Calorimetric investigations of hydrogen-bonded liquid crystal binary mixtures. J Therm Anal Calorim. 2018;134:1799–822. https://doi.org/10.1007/s10973-018-7688-7.
Kato T, Uchida J, Ichikawa T, Sakamoto T. Functional liquid crystals towards the next generation of materials. Angew Int Ed. 2018;57:4355–71. https://doi.org/10.1002/anie.201711163.
Kato T, Kato T, Fréchet JMJ, Uryu T, Kaneuchi F, Jin C, et al. Hydrogen-bonded liquid crystals built from hydrogen-bonding donors and acceptors Infrared study on the stability of the hydrogen bond between carboxylic acid and pyridyl moieties. LiqCryst. 2006;33:1429–37. https://doi.org/10.1080/02678290601119807.
Kato T, Frechet JM, Wilson PG, Saito T, Uryu T, Fujishima A, Jin C, Kaneuchi F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenicbipyridyl compounds through complexation between hydrogen-bond donor and acceptor moieties. Chem Mater. 2002;5:1094–100.
Navard P, Cox R. DCS study of smectic phase transitions. Mol Cryst Liq Cryst. 1984;102:265–9. https://doi.org/10.1080/01406568408070538.
Gopunath AJ, Chitravel T, Kavitha C, Prabu NPS, Madhu Mohan MLN. Thermal, optical, and dielectric analysis of hydrogen-bonded liquid crystals formed by adipic and alkyloxy benzoic acids. Mol Cryst Liq Cryst. 2014;592:63–81. https://doi.org/10.1080/15421406.2013.839315.
Chitravel T, Mohan MLNM. Occurrence of ambient temperature and reentrant smectic ordering in an intermolecular hydrogen bonding between alkyl aniline and alkoxy benzoic acids. Mol Cryst Liq Cryst. 2010;524:131–43. https://doi.org/10.1080/15421406.2010.484616.
Gopunath AJ, Chitravel T, Kavitha C, Prabu NPS, Madhu Mohan MLN. Double hydrogen bonded liquid crystals formed by glutaric Acid. Mol Cryst Liq Cryst. 2013;574:19–32.
Pongali Sathya Prabu N, Vijayakumar VN, Madhu Mohan MLN. Thermal and dielectric studies of self-assembly systems formed by hydroquinone and alkyloxy benzoic acids. Phys B Condens Matter. 2011;406:1106–13.
Acknowledgements
The infrastructural support provided by Centre for Research, Bannari Amman Institute of Technology is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chandrasekar, G., Balaji, R. & Pongali Sathya Prabu, N. Differential scanning calorimetric investigations of binaries formed between thermotropic liquid crystals. J Therm Anal Calorim (2024). https://doi.org/10.1007/s10973-024-13047-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10973-024-13047-1