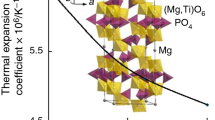
Abstract
The new polycrystalline phases of variable composition \({\mathrm{Na}}_{3}{\mathrm{Cr}}_{2}({\mathrm{AsO}}_{4}{)}_{\text{x}}({\mathrm{PO}}_{4}{)}_{3-\text{x}}\) were synthesized by co-precipitation of metal salts, arsenic acid, and ammonium hydrophosphate from an aqueous solution, followed by heat treatment. The obtained samples were examined using XRD, TG − DSC, SEM, and microprobe analysis. The \({\mathrm{Na}}_{3}{\mathrm{Cr}}_{2}({\mathrm{AsO}}_{4}{)}_{\text{x}}({\mathrm{PO}}_{4}{)}_{3-\text{x}}\) solid solution (1.75 ≤ x ≤ 3.0) exhibits dimorphism at elevated temperatures: low-temperature modification with garnet structure is obtained at 873–923 K and high-temperature rhombohedral modification at 1084–1317 K. The thermal expansion property of a low-temperature solid solution was studied in the temperature range of 143–473 K. The samples of solid solution expand isotropically. As the content of phosphorus in the solid solution increases, the coefficients of linear thermal expansion are raised.
Graphical abstract









Similar content being viewed by others
References
Sickafus KE, Melcher CL, Flynn-Hepford MI, et al. Crystal chemistry of rare-earth containing garnets: prospects for high configurational entropy. J Solid State Chem. 2022. https://doi.org/10.1016/j.jssc.2022.122997.
Milam-Guerrero J, Neer AJ, Melot BC. Crystal chemistry and competing magnetic exchange interactions in oxide garnets and spinels. J Solid State Chem. 2019;274:1–9.
Xing G, Zhu H, Zhuang A, et al. Doped superior garnet electrolyte toward all-solid-state Li metal batteries. Phys Open. 2022. https://doi.org/10.1016/j.jssc.2019.02.007.
Kuganathan N, Chroneos A. Atomic-scale studies of garnet-type Mg3Fe2Si3O12: defect chemistry, diffusion and dopant properties. J Power Sources Adv. 2020. https://doi.org/10.1016/j.powera.2020.100016.
Yamazaki Y, Miyake S, Akimoto K, et al. Effect of Ga2O3 addition on the properties of garnet-type Ta-doped Li7La3Zr2O12 solid electrolyte. Batteries. 2022. https://doi.org/10.3390/batteries8100158.
Sun Q, Chen X, Xie J, et al. Nano-structured Li1.3Al0.3Ti1.7(PO4)3 coated LiCoO2 enabling compatible interface with ultrathin garnet-based solid electrolyte for stable Li metal battery. Mater Today Nano. 2022. https://doi.org/10.1007/s00339-023-06796-7.
Yang X, Adair KR, Gao X, et al. Recent advances and perspectives on thin electrolytes for high-energy-density solid-state lithium batteries. Energy Environ Sci. 2021. https://doi.org/10.1039/D0EE02714F.
Han Y, Wang S, Liu H, et al. A novel Al3+ modified Li6CaLa2Sb2O12:Mn4+ far-red-emitting phosphor with garnet structure for plant cultivation. J Lumin. 2020;221:117031.
Huang D, Liang S, Chen D, et al. An efficient garnet-structured Na3Al2Li3F12:Cr3+ phosphor with excellent photoluminescence thermal stability for near-infrared LEDs. Chem Eng J. 2021. https://doi.org/10.1016/j.cej.2021.131332.
Kumar Mishra N, Upadhyay MM, Kumar S, et al. Efficient dual mode emission in Ce3+/Yb3+/Er3+ doped yttrium aluminium gallium garnet for led device and optical thermometry. Spectrochim Acta A Mol Biomol Spectrosc. 2022. https://doi.org/10.1016/j.saa.2022.121664.
Jamaludin NFA, Muthusamy K, Isa NN, et al. Use of spent garnet in industry: a review. Mater Today Proc. 2022. https://doi.org/10.1016/j.matpr.2021.02.210.
Muttashar HL, Ali NB, Mohd Ariffin MA, et al. Microstructures and physical properties of waste garnets as a promising construction materials. Case Stud Constr Mater. 2018. https://doi.org/10.1016/j.cscm.2017.12.001.
Stefanovsky SV, Yudintsev SV, Vinokurov SE, et al. Chemical-technological and mineralogical-geochemical aspects of the radioactive waste management. Geochem Int. 2016. https://doi.org/10.1134/S001670291613019X.
Alderton D. Garnets. In: Elias S, Alderton D editors. Elsevier. London 2021; 350–357.
Schwarz H, Schmidt L. Neue Verbindungen mit Granatstruktur. IV. Arsenate des Typs {Na3}[M2III](As3)O12. Z Anorg Allg Chem. 1972; 387: 31–42.
Winand J-M, Rulmont A, Tarte P. Synthese et etude de nouveaux arseniates (MI)3(NIII)2(AsO4)3 et de solutions solides (MI)3(NIII)2(AsO4)x(PO4)3–x (M = Li, Na; N = Fe, Sc, In, Cr). J Solid State Chem. 1990. https://doi.org/10.1016/0022-4596(90)90068-9.
Kouass S, Bouzemi B, Boughzala H. Garnet-type Li2.44K0.56Cr2(AsO4)3. Acta Crystallogr., Sect. E: Struct. Rep. Online. 2006; doi:https://doi.org/10.1107/S1600536806004788.
Khorari S, Rulmont A, Tarte P. Influence of cationic substitutions in Na3Fe2(AsO4)3: transition from the garnet to the alluaudite structure. J Solid State Chem. 1998. https://doi.org/10.1006/jssc.1997.7727.
Khorari S, Rulmont A, Tarte P. The arsenates NaCa2M2+2(AsO4)3 (M2+=Mg, Ni, Co): influence of cationic substitutions on the garnet-alluaudite polymorphism. J Solid State Chem. 1997. https://doi.org/10.1006/jssc.1997.7379.
Drebushchak VA. Thermal expansion of solids: review on theories. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-020-09370-y.
Anantharamulu N, Koteswara RK, Rambabu G, et al. A wide-ranging review on Nasicon type materials. J Mater Sci. 2011. https://doi.org/10.1007/s10853-011-5302-5.
Pet’kov VI. Complex phosphates formed by metal cations in oxidation states I and IV. Russ Chem Rev. 2012. https://doi.org/10.1070/RC2012v081n07ABEH004243.
Li H, Xu HZ, Wang YY, Zhou CL, et al. Preparation and properties of NZP family ceramics. Solid State Phenom. 2018. https://doi.org/10.4028/www.scientific.net/SSP.281.450.
Avdeev M. Crystal chemistry of NaSICONs: ideal framework, distortion, and connection to properties. Chem Mater. 2021. https://doi.org/10.1021/acs.chemmater.1c02695.
Singh B, Wang Z, Park S, et al. Chemical map of NaSiCON electrode materials for sodium-ion batteries. J Mater Chem. 2021. https://doi.org/10.26434/chemrxiv.13135811.
Yang Z, Tang B, Xie Z. NASICON-Type Na3Zr2Si2PO12 solid-state electrolytes for sodium batteries. ChemElectroChem. 2021. https://doi.org/10.1002/celc.202001527.
Stenina IA, Yaroslavtsev AB. Nanomaterials for lithium-ion batteries and hydrogen energy. Pure Appl Chem. 2017. https://doi.org/10.1515/pac-2016-1204.
Hou M, Liang F, Chen K, et al. Challenges and perspectives of NASICON-type solid electrolytes for all-solid-state lithium batteries. Nanotechnology. 2020. https://doi.org/10.1088/1361-6528/ab5be7.
Jian Z, Hu Y-S, Ji X, Chen W. NASICON-structured materials for energy storage. Adv Mater. 2017. https://doi.org/10.1002/adma.201601925.
Ananthanarayanan A, Ambashta RD, Sudarsan V, et al. Structure and short time degradation studies of sodium zirconium phosphate ceramics loaded with simulated fast breeder (FBR) waste. J Nucl Mater. 2017. https://doi.org/10.1016/j.jnucmat.2017.01.054.
Wang Y, Zhou Y, Tong N, et al. Crystal structure, mechanical and thermophysical properties of Ca0.5Sr0.5Zr4−xSnxP6O24 ceramics. J Alloys Compd. 2019. https://doi.org/10.1016/j.jallcom.2018.12.379.
Pet’kov VI, Sukhanov MV, Ermilova MM, et al. Development and synthesis of bulk and membrane catalysts based on framework phosphates and molybdates. Russ J Appl Chem. 2010. https://doi.org/10.1134/S1070427210100022.
Yamamoto K, Abe Y. Enhanced catalytic activity of microporous glass-ceramics with a skeleton of NASICON-type copper(I) titanium phosphate crystal. Mater Res Bull. 2000. https://doi.org/10.1016/S0025-5408(00)00206-3.
Zhukova AI, Asabina EA, Kharlanov AN, et al. Novel complex titanium NASICON-type phosphates as acidic catalysts for ethanol dehydration. Catalysts. 2023. https://doi.org/10.3390/catal13010185.
Pet’kov VI, Lavrenov DA, Kovalsky AM. Synthesis, characterization and thermal expansion of the zinc-containing phosphates with the mineral-like framework structures. J Therm Anal Calorim. 2020. https://doi.org/10.1007/s10973-019-08624-8.
Daneshwaran B, Triveni RM, Sathasivam PK. Influence of tin substitution on negative thermal expansion of K2Zr2-xSnxP2SiO12 (x = 0–2) phosphosilicates ceramics. Ceram Int. 2020. https://doi.org/10.1016/j.ceramint.2020.02.181.
Liu Y, Molokeev MS, Liu Q, Xia Z. Crystal structure, phase transitions and thermal expansion properties NaZr2(PO4)3 - SrZr4(PO4)6 solid solutions. Inorg Chem Front. 2018. https://doi.org/10.1039/C7QI00782E.
Bohre A, Avasthi K, Pet’kov VI. Vitreous and crystalline phosphate high level waste matrices: Present status and future challenges. J Ind Eng Chem. 2017. https://doi.org/10.1016/j.jiec.2017.01.032.
Pet’kov VI, Asabina EA, Lukuttsov AA, et al. Immobilization of cesium into mineral-like matrices of tridymite, kosnarite, and langbeinite structure. Radiochemistry. 2015. https://doi.org/10.1134/S1066362215060119.
Pet’kov VI, Asabina EA, Loshkarev V, Sukhanov M. Systematic investigation of the strontium zirconium phosphate ceramic form for nuclear waste immobilization. J Nucl Mater. 2016. https://doi.org/10.1016/j.jnucmat.2016.01.016.
Wang J, Wei Y, Wang J, et al. Simultaneous immobilization of radionuclides Sr and Cs by sodium zirconium phosphate type ceramics and its chemical durability. Ceram Int. 2022. https://doi.org/10.1016/j.ceramint.2022.01.147.
Kim Y-I, Izumi F. Structure refinements with a new version of the Rietveld-refinement program RIETAN. J Ceram Soc Jpn. 1994. https://doi.org/10.2109/JCERSJ.102.401.
Rietveld HM. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967. https://doi.org/10.1107/s0365110x67000234.
Acknowledgements
This work was supported by the Russian Science Foundation grant № 23-23-00044, https://rscf.ru/project/23-23-00044/.
Funding
This study was funded by the Russian Science Foundation, 23-23-00044, Vladimir I Pet*kov.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pet’kov, V.I., Piaterikov, E.A. & Fukina, D.G. Synthesis, phase transitions, and thermal expansion in arsenate-phosphate solid solution with garnet structure. J Therm Anal Calorim 148, 11569–11576 (2023). https://doi.org/10.1007/s10973-023-12514-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12514-5