Abstract
The development of new materials called geopolymers is described, which at the turn of the nineties brought a new state of the art in material design through the so-called wet process resulting in a specific amorphous state. The classical configuration of glasses prepared by quenching is used for a joint appraisal and judgment. We can use the comparison and description of the known form of organic polymers with the so-called mers-structure. The formation involves a sol–gel polycondensation chemical reaction also known in the case of organic polymers. The formation is described using aluminosilicate oxide in IV-fold coordination with alkaline polysilicates to form polymeric Si–O–Al chains through amorphous to semi- and hypo-crystalline three-dimensional silico-aluminate structures. The revision of structural units and their interconnection is evaluated, and it turns out that the common factor of the multiparty description is the existence of bridging and non-bridging oxygen. The review provides a detailed overview of opinion while reminding that the historical origin of the field falls within the purview of the JTAC journal.



Similar content being viewed by others
References
Davidovits J. Geopolymers. J Therm Anal. 1991;37(8):1633–56.
Koga N, Strnad Z, Sestak J, Strnad J. Thermodynamics of non-bridging oxygen in silica bio-compatible glass-ceramics—mimetic material for the bone tissue substitution. J Therm Anal Calorim. 2003;71(3):927–37.
Davidovits J, Davidovics M. Geopolymer: room-temperature ceramic matrix for composites. In: Proceedings of the 12th annual conference on composites and advanced ceramic materials: ceramic engineering and science proceedings. 1988, pp 835–41
Davidovits J. Geopolymers and geopolymeric materials. J Therm Anal. 1989;35(2):429–41.
Barrer RM. Some researched on silicates: mineral synthesis and metamorphosis. Trans Br Ceram Soc. 1957;56:155–61.
Gluchovskij VD. Gruntosilikaty. Kiev: Gosstrojizdat; 1959.
Knight CTG, Balec RJ, Kinrade SD. The structure of silicate anions in aqueous alkaline solutions. Angew Chem Int Ed. 2007;46(43):8148–52.
Toop GW, Somis CS. Some new ionic concepts of silicate slags. Can Metall Q. 1962;1(2):129–52.
Masson CR. Anionic constitution of glass-forming melts. J Non-Cryst Solids. 1977;25(1):1–41.
Těmkin M. Mixtures of fused salts as ionic solutions. Acta Physicoch URSS. 1945;20:4511–9.
Davidovits J. Geopolymers: ceramic-like inorganic polymers. J Ceram Sci Technol. 2017;8(3):335–50.
Kriven WM, Bell JL, Gordon M. Microstructure and microchemistry of fully-reacted geopolymers and geopolymer matrix composites. In: International symposium on recent advances in ceramic matrix composites. Nashville, 2003, pp 227–50.
Rahier H, VanMele B, Biesemans M, Wastiels J, Wu X. Low-temperature synthesized aluminosilicate glasses. 1. Low-temperature reaction stoichiometry and structure of a model compound. J Mater Sci. 1996;31(1):71–9.
Rowles MR, O’Connor BH. Chemical and structural microanalysis of aluminosilicate geopolymers synthesized by sodium silicate activation of metakaolinite. J Am Ceram Soc. 2009;92(10):2354–61.
Kriven WM. Inorganic polysialates or “geopolymers.” Am Ceram Soc Bull. 2010;89(4):31–4.
Šestak J, Foller B. Some aspects of composite inorganic polysialates. J Therm Anal Calorim. 2012;108(2):511–7.
Provis JL, Palomo A, Shi CJ. Advances in understanding alkali-activated materials. Cem Concr Res. 2015;78:110–25.
Mysen BO, Shang J. Evidence from olivine/melt element partitioning that nonbridging oxygen in silicate melts are not equivalent. Geochim Cosmochim Acta. 2005;69(11):2861–75.
Machacek J, Gedeon O, Liska M. Group connectivity in binary silicate glasses. J Non-Cryst Solids. 2006;352(21–22):2173–9.
Greaves GN, Sen S. Inorganic glasses, glass-forming liquids and amorphizing solids. Adv Phys. 2007;56(1):1–166.
Šestak J, Strnad Z, Strnad J, Holecek M, Koga N. Biomedical thermodynamics and implantology aspects of biocompatible glass-ceramics and otherwise modified inorganic materials and surfaces. In: 9th Conference of the European-society-of-glass-science-and-technology/annual meeting of the international-commission-on-glass. Trencin, SLOVAKIA; 2008, pp 329.
Závěta K, Šesták J. Structure, bridging oxygen and magnetic properties of Fe-rich oxide glasses. In: 21st International congress on glass. Prague: Dům techniky ČVTS; 1977, pp 399.
Šesták J. Nonbridging oxygen in silica biocompatible glass ceramics and magnetic properties of Fe2O3 added borate glasses. In: Šesták J, Holeček M, Málek J, editors. Some thermodynamic, structural and behavioral aspects of glassy and amorphous materials. Plzeň: OPS-ZČU Plzeň; 2009. p. 128–51.
Komnitsas K, Zaharaki D. Geopolymerisation: a review and prospects for the minerals industry. Min Eng. 2007;20(14):1261–77.
Khale D, Chaudhary R. Mechanism of geopolymerization and factors influencing its development: a review. J Mater Sci. 2007;42(3):729–46.
Duxson P, Fernández-Jiménez A, Provis JL, Lukey GC, Palomo A, van Deventer JSJ. Geopolymer technology: the current state of the art. J Mater Sci. 2007;42(9):2917–33.
Li C, Sun HH, Li LT. A review: the comparison between alkali-activated slag (Si plus Ca) and metakaolin (Si plus Al) cements. Cem Concr Res. 2010;40(9):1341–9.
Lopes AC, Martins P, Lanceros-Mendez S. Aluminosilicate and aluminosilicate based polymer composites: present status, applications and future trends. Prog Surf Sci. 2014;89(3–4):239–77.
Palomo A, Krivenko P, Garcia-Lodeiro I, Kavalerova E, Maltseva O, Fernandez-Jimeneza A. A review on alkaline activation: new analytical perspectives. Mater Constr. 2014;64(315):e022.
Nikolov A, Nugteren H, Rostovsky I. Optimization of geopolymers based on natural zeolite clinoptilolite by calcination and use of aluminate activators. Constr Build Mater. 2020;243:118257.
Cong PL, Cheng YQ. Advances in geopolymer materials: a comprehensive review. J Traffic Transp Eng Engl Ed. 2021;8(3):283–314.
Castillo H, Collado H, Droguett T, Vesely M, Garrido P, Palma S. State of the art of geopolymers: a review. E-Polymers. 2022;22(1):108–24.
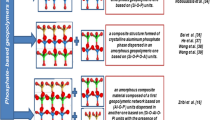
Zribi M, Baklouti S. Phosphate-based geopolymers: a critical review. Polym Bull. 2022;79(9):6827–55.
Longhi MA, Rodriguez ED, Walkley B, Eckhard D, Zhang ZH, Provis JL, et al. Metakaolin-based geopolymers: efflorescence and its effect on microstructure and mechanical properties. Ceram Int. 2022;48(2):2212–29.
Marvila MT, Azevedo ARG, Vieira CMF. Reaction mechanisms of alkali-activated materials, a review. Rev IBRACON Estrut Mater. 2021;14(3):e14309.
Šesták J, Koga N, Šimon P, Foller B, Roubíček P, Wu N-LN. Amorphous inorganic polysialates: geopolymeric composites and the bioactivity of hydroxyl groups. In: Šesták J, Šimon P, editors. Thermal analysis of micro, nano- and non-crystalline materials: transformation, crystallization, kinetics and thermodynamics. Dordrecht: Springer; 2013. p. 441–60.
Šestak J. Non-bridging oxygen in silica biocompatible glasses, ceramics, polysialates and geopolymers. In: Sestak J, editor. Thermal analysis and thermodynamic properties of solids. 2nd ed. Amsterdam: Elsevier; 2021. p. 437–58.
Šestak J. Miracle of reinforced states of matter. Glasses: ancient and innovative materials for the third millennium. J Therm Anal Calorim. 2000;61(1):305–23.
Collins LE. Overview of rapid solidification technology. Can Metallu Q. 1986;25(1):59–72.
Kramer MJ, Mecco H, Dennis KW, Vargonova E, McCallum RW, Napolitano RE. Rapid solidification and metallic glass formation—experimental and theoretical limits. J Non-Cryst Solids. 2007;353(32–40):3633–9.
Šesták J. The art and horizont of non-equilibriated and disordered states and the new glassy phase rapid formation techniques. Glass Sci Technol. 1997;70(1):153–63.
Kawazoe Y, Carow-Watamura U, Louzguine DV. A brief introduction to bulk metallic glasses: datasheet from condensed matter. In: Carow-Watamura U, editor. Phase diagrams and physical properties of nonequilibrium alloys, vol. 37C3. Berlin: Springer; 2013. https://doi.org/10.1007/978-3-662-57920-6_1.
Illeková E, Šesták J. Crystallization of metallic micro-, nano-, and non-crystalline alloys. In: Šesták J, Šimon P, editors. Thermal analysis of micro, nano- and non-crystalline materials: transformation, crystallization, kinetics and thermodynamics. Dordrecht: Springer; 2013. p. 257–89.
Prasad N. Introduction to metallic glasses. Metallic glass–based nanocomposites. Boca Raton: CRC Press; 2019.
Queiroz CA, Šestak J. Aspects of the non-crystalline state. Phys Chem Glass Eur J Glass Sci Technol Part B. 2010;51(3):165–72.
Kozmidis-Petrovic A, Šestak J. Forty years of the Hruby glass-forming coefficient via DTA when comparing other criteria in relation to the glass stability and vitrification ability. J Therm Anal Calorim. 2012;110(2):997–1004.
Šestak J, Šestakova V, Třiska A, Zavěta K. Glass-formation, phase-relations and magnetic-properties of the splat-quenched system of laser-melted (FE, MN)2O3-(BI, B)2O3. J Therm Anal Calorim. 1988;33(3):789–95.
Iordanova R, Dimitriev Y, Dimitrov V, Kassabov S, Klissurski D. Glass formation and structure in the system MoO3–Bi2O3–Fe2O3. J Non-Cryst Solids. 1998;231(3):227–33.
Šestak J. Use of phenomenological kinetics and the enthalpy versus temperature diagram (and its derivative—DTA) for a better understanding of transition processes in glasses. Thermochim Acta. 1996;280:175–90.
Schumacher O, Marvel CJ, Kelly MN, Cantwell PR, Vinci RP, Rickman JM, et al. Complexion time-temperature-transformation (TTT) diagrams: opportunities and challenges. Curr Opin Solid State Mater Sci. 2016;20(5):316–23.
Šesták J. Thermal analysis and thermodynamic properties of solids. 2nd ed. Amsterdam: Elsevier; 2021.
Machaček J, Gedeon O. Q-species in alkali-disilicate glasses. Ceram Silik. 2003;47(2):45–9.
Liška M, Klement R, Machaček J, Gedeon O. Inverse thermodynamic modelling of glass from Raman spectroscopical and molecular dynamics results. Phys Chem Glasses. 2005;46(2):108–11.
Liška M, Macháček J, Gedeon O. Molecular dynamics of the Na2O–MgO–SiO2 system. Glass Sci Technol. 2004;77(1):267.
Zaharaki D, Komnitsas K, Perdikatsis V. Use of analytical techniques for identification of inorganic polymer gel composition. J Mater Sci. 2010;45(10):2715–24.
Gasca-Tirado JR, Manzano-Ramírez A, Rivera Muñoz EM, Velázquez-Castillo R, Apátiga-Castro M, Nava R, et al. Ion exchange in geopolymers. In: Selcan K, editor., et al., New trends in ion exchange studies. Rijeka: IntechOpen; 2018.
Virgo D, Mysen BO, Kushiro I. Anionic constitution of 1-atmosphere silicate melts—implications for the structure of igneous melts. Science. 1980;208(4450):1371–3.
Mysen B, Richet P. Silicate glasses and melts. 2nd ed. Amsterdam: Elsevier; 2019.
Steevels IM. Neue erkenntnisse uber die struktur des glases. Philips Tech Rundschau. 1960;9–10:337.
Hench LL. Bioactive ceramics. Ann N Y Acad Sci. 1988;523(1):54–71.
Masson CR. Ionic equilibria in liquid silicates. J Am Ceram Soc. 1968;51(3):134–0.
Hench LL. Glasses and genes: a forecast for the future. Glass Sci Technol. 1997;70:439–48.
Hench LL. Life and death: The ultimate phase transformation. Thermochim Acta. 1996;280:1–13.
Šestak J, Liška M, Hubik P. 12—Oxide glass structure, bridging oxygen and feasible magnetic and structural properties due to the addition of Fe/Mn oxides. In: Sestak J, Mares JJ, Hubik P, editors. Glassy, amorphous and nano-crystalline materials: thermal physics, analysis, structure and properties. Dordrecht: Springer; 2011.
Provis JL, Van Deventer JSJ. Geopolymers: structures, processing, properties and industrial applicatiosn. Sawston: Woodhead Publishing; 2009.
Mazen A, Han-Yong J. Geopolymers and other geosynthetics. Rijeka: Intechopen; 2020.
Davidovits J. Geopolymer chemistry and applications. 5th ed. Saint-Quentin: Geopolymer Institute; 2020.
Kóth J, Sinkó K. Geopolymer composites-in environmentally friendly aspects. Gels. 2023;9:196. https://doi.org/10.3390/gels9030196.
Šestak J, Černy R. Thermotics as an alternative nonequilibrium thermodynamic approach suitable for real thermoanalytical measurements: a short review. J Non-Equilib Thermodyn. 2022;47:233–40.
Plasencia G, Jaramillo D. Thermochemistry in materials processing. Berlin: Springer; 2017.
Delhaes P. Materials and thermodynamics (materials science). Hoboken: Wiley; 2017.
Klimm D. Thermal analysis and thermodynamics in materials science. Berlin: De Gruyter; 2022.
Ebeid EZ, Zakaria M. Thermal analysis: from introductory fundamentals to advanced applications. Amsterdam: Elsevier; 2021.
Acknowledgements
This research has been supported by the Technology Agency of the Czech Republic under project No. FW01010229 and by the specific research project of the University of West Bohemia in Pilsen, NaturTech4 SGS-2022-921.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Šesták, J., Kočí, V., Černý, R. et al. Thirty years since J. Davidovits introduced geopolymers considered now as hypo-crystalline materials within the mers-framework and the effect of oxygen binding: a review. J Therm Anal Calorim 148, 10455–10463 (2023). https://doi.org/10.1007/s10973-023-12312-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-023-12312-z