Abstract
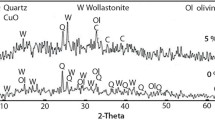
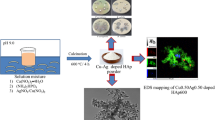
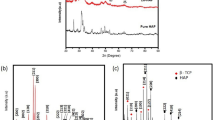
This work aims to obtain antibacterially active wollastonite by synthesizing calcium silicate hydrates in dense samples and, subsequently, by calcinating them at a low temperature. For the synthesis of calcium silicate hydrates, cylindrical pellets (h = 4 mm and d = 40 mm) of calcium oxide and silicon dioxide (CaO/SiO2 = 0.66) were prepared. Then, the pellets were treated in an autoclave under saturated steam pressure at a temperature of 200 °C for 4–72 h. It was determined that, after 4 h of synthesis, a lower-basicity calcium silicate hydrate—Z-phase—was formed. By prolonging the synthesis to 16–72 h, Z-phase became metastable and recrystallized to gyrolite. The samples formed after 4 h and 72 h of synthesis were used for the adsorption of copper ions. It should be emphasized that the adsorption capacity of the sample obtained after 4 h (140 mg Cu2+ g−1) was higher than that of the sample formed after 72 h (130 mg Cu2+ g−1). The samples with intercalated copper ions were used for the synthesis of wollastonite. Wollastonite was synthesized in a high-temperature furnace at a temperature of 900 °C for 1 h. It should be noted that, during calcination, together with wollastonite, copper oxide was formed in all the investigated samples. The results of the agar diffusion assay showed that wollastonite synthesized using dense calcium silicate hydrate samples demonstrated antibacterial activity against the investigated Bacillus cereus, Escherichia coli, and Staphylococcus aureus bacteria.









Similar content being viewed by others
References
Yan G, et al. Structural and electrochemical properties of wollastonite-based chemically bonded phosphate ceramic coatings. Ceram Int. 2021;47:9961–70. https://doi.org/10.1016/j.ceramint.2020.12.141.
Zulumyan N, Mirgorodski A, Isahakyan A, Beglaryan H, Gabrielyan A, Terzyan A. A low-temperature method of the β-wollastonite synthesis. J Therm Anal Calorim. 2015;122:97–104. https://doi.org/10.1007/s10973-015-4752-4.
Saadaldin SA, Rizkalla AS. Synthesis and characterization of wollastonite glassceramics for dental implant applications. Dent Mater. 2014;30:364–71. https://doi.org/10.1016/j.dental.2013.12.007.
Mahdy MA, et al. Influence of silicon carbide on structural, optical and magnetic properties of Wollastonite/Fe2O3 nanocomposites. Ceram Int. 2021;47:12047–55. https://doi.org/10.1016/j.ceramint.2021.01.048.
Kubátová D, et al. Formation of belite-based binder from waste materials. J Therm Anal Calorim. 2020;142:1625–33. https://doi.org/10.1007/s10973-020-10252-6.
Somtürk SM, et al. Effect of wollastonite extender on the properties of exterior acrylic paints. Prog Org Coating. 2016;93:34–40. https://doi.org/10.1016/j.porgcoat.2015.12.014.
Obeid MM. Crystallization of synthetic wollastonite prepared from local raw materials. Int J Mater Chem. 2014;4:79–87. https://doi.org/10.5923/j.ijmc.20140404.01.
Young JF, Dimitry S. Electrical properties of chemically bonded ceramic insulators. J Am Ceram Soc. 1990;73:2775–8. https://doi.org/10.1111/j.1151-2916.1990.tb06765.x.
Soliman AM, Nehdi ML. Effect of natural wollastonite microfibers on early-age behavior of UHPC. J Mater Civ Eng. 2012;24:816–24. https://doi.org/10.1061/(ASCE)MT.1943-5533.0000473.
U. National Minerals Information Center, Wollastonite Data Sheet – Mineral Commodity Summaries 2020.
Wahab MA, et al. The use of wollastonite to enhance the mechanical properties of mortar mixes. Constr Build Mater. 2017;152:304–9. https://doi.org/10.1016/j.conbuildmat.2017.07.005.
He Z, et al. Effect of wollastonite microfibers as cement replacement on the properties of cementitious composites: a review. Constr Build Mater. 2020;262:119920. https://doi.org/10.1016/j.conbuildmat.2020.119920.
Vakalova TV, Pogrebenkov VM, Karionova NP. Solid-phase synthesis of wollastonite in natural and technogenic siliceous stock mixtures with varying levels of calcium carbonate component. Ceram Int. 2016;42:16453–62. https://doi.org/10.1016/j.ceramint.2016.06.060.
Abd Rashid R, et al. In-vitro bioactivity of wollastonite materials derived from limestone and silica sand. Ceram Int. 2014;40:6847–53. https://doi.org/10.1016/j.ceramint.2013.12.004.
Zhu LZ, Sohn HY, Bronson TM. Flux growth of 2M-wollastonite crystals for the preparation of high aspect ratio particles. Ceram Int. 2014;40:5973–82. https://doi.org/10.1016/j.ceramint.2013.11.045.
Sasikumar S, Ravy L. Influence of needle-like morphology on the bioactivity of nanocrystalline wollastonite—an in vitro study. Int J Nanomed. 2015;10:129–36. https://doi.org/10.2147/IJN.S79986.
Luyt AS, Dramićanin MD, Antić Ž, Djoković V. Morphology, mechanical and thermal properties of composites of polypropylene and nanostructured wollastonite filler. Polym Test. 2009;28:348–56. https://doi.org/10.1016/j.polymertesting.2009.01.010.
Arcos D, Vallet-Regi M. Sol-gel silica-based biomaterials and bone tissue regeneration. Acta Biomater. 2010;6:2874–88. https://doi.org/10.1016/j.actbio.2010.02.012.
Li X, Chang J. Synthesis of wollastonite single crystal nanowires by a novel hydrothermal route. Chem Lett. 2004;33:1458–9. https://doi.org/10.1246/cl.2004.1458.
Pei LZ, et al. A green and facile route to synthesize calcium silicate nanowires. Mater Char. 2010;61:1281–5. https://doi.org/10.1016/j.matchar.2010.07.002.
Iljina A, et al. The stability of formed CaF2 and its influence on the thermal behavior of C-S–H in CaO–silica gel waste-H2O system. J Therm Anal Calorim. 2017;127:221–8. https://doi.org/10.1007/s10973-016-5412-z.
Lin K, Chang J, Lu J. Synthesis of wollastonite nanowires via hydrothermal microemulsion methods. Mater Lett. 2006;60:3007–10. https://doi.org/10.1016/j.matlet.2006.02.034.
Kong N, Lin K, Li H, Chang J. Synergy effects of copper and silicon ions on stimulation of vascularization by copper-doped calcium silicate. J Mater Chem B. 2014;2:1100–10. https://doi.org/10.1039/c3tb21529f.
Azeena S, et al. Antibacterial activity of agricultural waste derived wollastonite doped with copper for bone tissue engineering. Mater Sci Eng. 2017;71:1156–65. https://doi.org/10.1016/j.msec.2016.11.118.
Li HC, Wang DG, Chen CZ. Effect of zinc oxide and zirconia on structure, degradability and in vitro bioactivity of wollastonite. Ceram Int. 2015;41:10160–9. https://doi.org/10.1016/j.ceramint.2015.04.117.
Wu C, Ramaswamy Y, Soeparto A, Zreiqat H. Incorporation of titanium into calcium silicate improved their chemical stability and biological properties. J Biomed Mater Res A. 2008;86:402–10. https://doi.org/10.1002/jbm.a.31623.
Zakaria MY, et al. Incorporation of wollastonite bioactive ceramic with titanium for medical applications: an overview. Mater Sci Eng C. 2019;97:884–95. https://doi.org/10.1016/j.msec.2018.12.056.
Ramaswamy Y, et al. The responses of osteoblasts, osteoclasts and endothelial cells to zirconium modified calcium silicate-based ceramic. Biomaterials. 2008;29:4392–402. https://doi.org/10.1016/j.biomaterials.2008.08.006.
Valenzuela F, et al. Adsorption of pollutant ions from residual aqueous solutions onto nano-structured calcium silicate. J Chil Chem Soc. 2013;58:1744–9. https://doi.org/10.4067/S0717-97072013000200023.
Panday KK, Prasad G, Singh VN. Use of wollastonite for the treatment of Cu(II) rich effluents. Water Air Soil Pollut. 1986;27:287–96. https://doi.org/10.1007/BF00649410.
Reddy MV, Pathak M. Sol-gel combustion synthesis of Ag doped CaSiO3: in vitro bioactivity, antibacterial activity and cytocompatibility studies for biomedical applications. Mater Technol. 2018;33:38–47. https://doi.org/10.1080/10667857.2017.
Kalaivani S, Kishore Singh R, Ganesan V, Kannan S. Effect of copper (Cu2+) inclusion on the bioactivity and antibacterial behavior of calcium silicate coatings on titanium metal. Mater Chem B. 2014;2:846–58. https://doi.org/10.1039/C3TB21522A.
Reddy MV, Pathak M. In vitro biological evaluations of Zn doped CaSiO3 synthesized by sol-gel combustion technique. J Inorg Organomet Polym. 2018;28:2187–95. https://doi.org/10.1007/s10904-018-0922-8.
Gineika A, Dambrauskas T, Baltakys K. Synthesis and characterisation of wollastonite with aluminium and fluoride ions. Ceram Int. 2021;47:22900–10. https://doi.org/10.1016/j.ceramint.2021.05.003.
Gineika A, Baltakys K, Dambrauskas T. The application of silica gel waste for the two-step synthesis of wollastonite in temperature range of 200–950 °C. J Therm Anal Calorim. 2019;38:2263–73. https://doi.org/10.1007/s10973-019-08481-5.
Baltakys K, Siauciunas R. The influence of γ-Al2O3 and Na2O on the formation of calcium silicate hydrates in the CaO–quartz–H2O system. Mater Sci. 2007;25:185–98.
Baltakys K, Siauciunas R. Gyrolite formation in CaO–SiO2 center dot nH(2)O-gamma-Al2O3–Na2O–H2O system under hydrothermal conditions. Pol J Chem. 2007;81(1):103–14.
Bankauskaite A, Baltakys K, Eisinas A, Zadaviciute S. Study on adsorption of heavy metal ions in wastewater by synthetic layered inorganic adsorbents. Desalin Water Treat. 2015;56:1576–86. https://doi.org/10.1080/19443994.2014.951074.
Viallis-Terrisse H, Nonat A, Petit JC. Zeta-potential study of calcium silicate hydrates interacting with alkaline cations. J Colloid Interf Sci. 2001;244:58–65. https://doi.org/10.1006/jcis.2001.7897.
Sayuri T, et al. Modeling of the adsorption behavior of Cs and Sr on calcium silicate hydrates. J Adv Concr Technol. 2021;19:1061–74.
Baltakys K, et al. The influence of hydrothermal synthesis conditions on gyrolite texture and specific surface area. Mater Struct. 2011;44:1687–701. https://doi.org/10.1617/s11527-011-9727-8.
Siauciunas R, Baltakys K. Formation of gyrolite during hydrothermal synthesis in the mixtures of CaO and amorphous SiO2 or quartz. Cem Concr Res. 2004;34:2029–36. https://doi.org/10.1016/j.cemconres.2004.03.009.
Kasperaviciute V, Baltakys K, Siauciunas R. The sorption properties of gyrolite for copper ions. Ceram Silik. 2008;5:295–101.
Kong L, et al. Preparation and characterization of slice-like Cu2(OH)3NO3 superhydrophobic structure on copper foil. Appl Surf Sci. 2008;254:7255–8. https://doi.org/10.1016/j.apsusc.2008.05.317.
Kociolek-Balawejder E, et al. Cu2O doped gel-type anion exchanger obtained by reduction of brochantite deposit and its antimicrobial activity. React Funct Polym. 2019;141:42–9. https://doi.org/10.1016/j.reactfunctpolym.2019.05.006.
Noyce JO, Michels H, Keevil CW. Potential use of copper surfaces to reduce survival of epidemic methicillin-resistant Staphylococcus aureus in the healthcare environment. J Hosp Infect. 2006;63:289–97. https://doi.org/10.1016/j.jhin.2005.12.008.
Radovanović N, et al. Tailoring the physico-chemical and antimicrobial properties of agar-based films by in situ formation of Cu-mineral phase. Eur Polym J. 2019;119:352–8. https://doi.org/10.1016/j.eurpolymj.2019.08.004.
Acknowledgments
This research was supported by the Research and Innovation Fund of Kaunas University of Technology (Grant No. PP59/2002).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eisinas, A., Dambrauskas, T., Eisinaite, V. et al. Synthesis of antibacterially active wollastonite by using dense calcium silicate hydrate samples. J Therm Anal Calorim 147, 14163–14174 (2022). https://doi.org/10.1007/s10973-022-11632-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-022-11632-w