Abstract
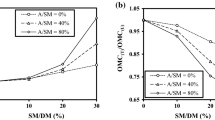
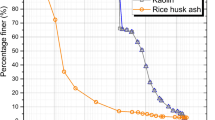
The effect of monoaluminum phosphate (Al(H2PO4)3) addition in the thermochemical process of a kaolinitic clay was studied and compared with the pure clay. Monoaluminum phosphate incorporation is of technological interest for the widely use of this material as an effective binder. From this point of view is important to clarify the processing strategy of kaolinitic clay-MAP based ceramics. This work comprised the characteristics of the obtained ceramics using differential thermal analysis, thermogravimetric analysis (TG), dilatometric analysis, X-ray powder diffraction with Rietveld refinement, mechanical resistance and textural properties. The addition of MAP did not affect the temperature of the kaolinite dehydroxilation (600–500 °C) but reduced this transformation as it was observed in TG curve. The amount of mullite decreased and cristobalite formation was stimulated with MAP presence in the samples fired at 1400 °C. Mullite cell parameters were not modified. The present study gives information for further clay-based materials designed with MAP. It can be concluded that the incorporation of low percentages of MAP in the potential formulation of technological ceramics would not imply important changes in the macroscopic properties of these type of ceramic materials.









Similar content being viewed by others
References
Chakraborty AK. Phase transformation of kaolinite clay. New Delhi: Springer; 2014.
Dondi M, Raimondo M, Zanelli C. Clays and bodies for ceramic tiles: reappraisal and technological classification. Appl Clay Sci. 2014;96:91–109.
Schroeder PA, Erickson G. Kaolin: from ancient porcelains to nanocomposites. Elements. 2014;10:177–82.
Konta J. Clay and man: clay raw materials in the service of man. Appl Clay Sci. 1995;10:275–335.
Andrini L, Gauna MR, Conconi MS, Suarez G, Requejo G, Aglietti EF, et al. Extended and local structural description of a kaolinitic clay, its fired ceramics and intermediates: an XRD and XANES analysis. Appl Clay Sci. 2016;124–125:39–45.
Torres Sánchez RM, Basaldella EI, Marco JF. The effect of thermal and mechanical treatments on kaolinite: characterization by XPS and IEP measurements. J Colloid Interface Sci. 1999;215:339–44.
Hernández MF, Violini MA, Serra MF, et al. Boric acid (H3BO3) as flux agent of clay-based ceramics, B2O3 effect in clay thermal behavior and resultant ceramics properties. J Therm Anal Calorim. 2020;139:1717–29.
Ondruška J, Csáki Š, Štubňa I, et al. Investigation of kaolin–quartz mixtures during heating using thermodilatometry and DC thermoconductometry. J Therm Anal Calorim. 2020;139:833–8.
Al-Shantir O, Csáki Š, Ondro T, et al. Mechanical-acoustic study of electroporcelain mixture made under different compression pressures. J Therm Anal Calorim. 2020;142(5):1759–66.
Chen D, He L, Shang S. Study on aluminum phosphate binder and related Al2O3–SiC ceramic coating. Mater Sci Eng. 2003;348:29–35.
Kingery WD. Fundamental study of phosphate bonding in refractories: I, literature review. J Am Ceram Soc. 1950;33:239–41.
Vippola M, et al. Structural characterization of aluminum phosphate binder. J Am Ceram Soc. 2000;83:1834–6.
Sahnoun RD, Bouaziz J. Sintering characteristics of kaolin in the presence of phosphoric acid binder. Ceram Int. 2012;38:1–7.
Luz AP, Gomes DT, Pandolfelli VC. High-alumina phosphate-bonded refractory castables: Al(OH)3 sources and their effects. Ceram Int. 2015;41:9041–50.
Hipedinger NE, Scian AN, Aglietti EF. Magnesia-phosphate bond for cold-setting cordierite-based refractories. Cem Concr Res. 2002;32:675–82.
Colonetti E, Hobold Kammer E, De Noni JA. Chemically-bonded phosphate ceramics obtained from aluminum anodizing waste for use as coatings. Ceram Int. 2014;40:14431–8.
Wang YS, Dai JG, Ding Z, Xu mass% Phosphate-based geopolymer: formation mechanism and thermal stability. Mater Lett. 2017;190:209–12.
Almanza RJM, Castillejos EAH, Acosta GFA, Flores VA. Microstructure and properties characterization of a new ceramic filter —CEFILPB. Mater Des. 1994;15:135–40.
Mocciaro A, Lombardi MB, Scian AN. Effect of raw material milling on ceramic proppants properties. Appl Clay Sci. 2018;153:90–4.
Morris JH, Perkins PG, Rose AEA, Smith WE. The chemistry and binding properties of aluminium phosphates. Chem Soc Rev. 1977;6:173–94.
Almanza RJM, Castillejos EAH, Acosta GFA, Flores VA. Microstructure and properties characterization of a new ceramic filter CEFILPB. Mater Des. 1994;15:135–40.
Zulkifly K, et al. 2020 Thermal exposure of fly ash-metakaolin blend geopolymer with addition of monoaluminum phosphate (MAP). In: IOP Conf. Ser.: Mater. Sci. Eng. https://doi.org/10.1088/1757-899X/864/1/012011.
Dominguez E, Cravero F. Los recursos de caolín de Chubut y Santa Cruz. Recur Miner Repub Argent. Zappettini E.O. Argentina: Instituto de Geología y Recursos Minerales. Secretaría de Estado de Geología y Minería de Argentina; 1999. pp. 1265–1272.
Rietveld HM. A profile refinement method for nuclear and magnetic structures. J Appl Crystallogr. 1969;2:65–71.
Le Bail A. Modelling the silica glass structure by the Rietveld method. J Non Cryst Solids. 1995;183:39–42.
Hernández MF, Conconi MS, Cipollone M, Herrera MS, Rendtorff NM. Ceramic behavior of ball clay with gadolinium oxide (Gd2O3) addition. Appl Clay Sci. 2017;146:380–7.
Xu X, et al. Microstructural evolution, phase transformation, and variations in physical properties of coal series kaolin powder compact during firing. Appl Clay Sci. 2015;115:76–86.
Okada K, Otsuka N, Ossaka J. Characterization of spinel phase formed in the kaolin-mullite thermal sequence. J Am Ceram Soc. 1986;69:C251–3.
Ptáček P, et al. The kinetics and mechanism of kaolin powder sintering I. The dilatometric CRH study of sinter-crystallization of mullite and cristobalite. Powder Technol. 2012;232:24–30.
Zanelli C, Raimondo M, Guarini G, Dondi M. The vitreous phase of porcelain stoneware: composition, evolution during sintering and physical properties. J Non Cryst Solids. 2011;357:3251–60.
Beck WR. Crystallographic inversions of the aluminum orthophosphate polymorphs and their relation to those of silica. J Am Ceram Soc. 1949;32:147–51.
Kumar P, Tiwari AN, Bhargava P. Effect of process parameters and binder concentration on mechanical properties of phosphate bonded alumina. Trans Indian Ceram Soc. 2013;72:130–5.
Butler MA, Dyson DJ. The quantification of different forms of cristobalite in devitrified alumino-silicate ceramic fibres. J Appl Crystallogr. 1997;30:467–75.
Madsen IC, Finney RJ, Flann RCA, Frost MT, Wilson BW. Quantitative analysis of high-alumina refractories using X-ray powder diffraction data and the rietveld method. J Am Ceram Soc. 1991;74:619–24.
Karlsson KH, Rönnlöf M. Property-composition relationships for potentially bioactive glasses. Glass Sci Technol (Frankfurt). 1998;71(5):141–5.
Herrera MS, Hernández MF, Cipollone M, Conconi MS, Rendtorff NM. Thermal behavior of samarium oxide—Ball clay mixtures for high macroscopic neutron capture cross section ceramic materials. Appl Clay Sci. 2019;168:125–35.
Schneider H, Schreuer J, Hildmann B. Structure and properties of mullite—a review. J Eur Ceram Soc. 2008;28:329–44.
Acknowledgements
This work was supported by CONICET. This work has been partially supported by Nano-Petro FONARSEC Project 2012 (ANPCyT). N.M.R. thanks FONCyT-BID PICT 2016-1193.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mocciaro, A., Conconi, M.S., Rendtorff, N.M. et al. Ceramic properties of kaolinitic clay with monoaluminum phosphate (Al(H2PO4)3) addition. J Therm Anal Calorim 144, 1083–1093 (2021). https://doi.org/10.1007/s10973-020-10488-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10488-2