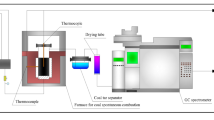
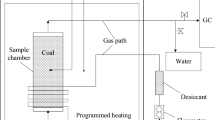
Abstract
Organic elastomers such as polyurethane are widely used as a filling material in underground coal mines. However, the risk of fire hazard in coal seams is increased due to the flammability of polyurethane. Laboratory tests illustrate that in a thermostatic reactor with initial temperature of 25 °C, the temperature during the production of polyurethane increases up to > 140 °C. Adiabatic oxidation experiments and TG–DTG analysis showed that the heating rate of the mixed samples of polyurethane and coal was higher than that of any single component in the initial oxidation stage. From the initial temperature to 100 °C, increasing the proportion of polyurethane was conducive to the acceleration of temperature rise. From 0.180 to 0.850 mm, the self-heating rate was inversely proportional to the particle size. At the initial stage, the low ventilation rate promoted the spontaneous combustion of mixed samples, but when the temperature is > 70 °C, an opposite trend was seen. Kinetic parameters suggested that the activation energy decreases with the introduction of polyurethane. According to the mathematical model, the variation of the spontaneous combustion period of coal and polyurethane was predicted.













Similar content being viewed by others
Abbreviations
- \(A\) :
-
Pre-exponential factor (s−1)
- \(E\) :
-
Activation energy (kJ mol−1)
- \(R\) :
-
Universal gas constant (J mol−1 K−1)
- \(C_{{\text{S}}}\) :
-
Constant in Starink formula
- \(G\left( a \right)\) :
-
Integral mechanism function
- \(Q\) :
-
Energy (J)
- \(R\left( T \right)\) :
-
Heat flow rate (J s−1 g−1)
- \(m\left( T \right)\) :
-
Sample mass (g)
- \(\omega \left( T \right)\) :
-
Sample moisture content (%)
- \(c_{{\text{s}}} \left( T \right)\) :
-
The specific heat capacity of the sample (J g−1 K−1)
- \(c_{{\text{w}}}\) :
-
The specific heat of water (J g−1 K−1)
- \(h\) :
-
The latent heat of evaporation of water (J g−1)
- \(m_{0}\) :
-
The initial mass of the sample (g)
- \(\omega_{0}\) :
-
The percentage of water that evaporates (%)
- \(A_{{\text{e}}}\) :
-
The heat dissipation surface area (m2)
- \(T_{{\text{e}}}\) :
-
Environment temperature (K)
- \(A_{{\text{i}}}\) :
-
The heated surface area (m2)
- \(T_{{\text{i}}}\) :
-
Instrument temperature (K)
- \(a\) :
-
Conversion degree (%)
- \(\alpha\) :
-
Coefficient of heat dispersion (W m−2 K−1)
- \(\beta\) :
-
Heating rate (K min−1)
- \(\lambda\) :
-
Coefficient of heat transfer (W m−2 K−1)
References
Song Z, Kuenzer C. Coal fires in China over the last decade: a comprehensive review. Int J Coal Geol. 2014;133:72–99. https://doi.org/10.1016/j.coal.2014.09.004.
Singh RVK. Spontaneous heating and fire in coal mines. Proc Eng. 2013;62:78–90. https://doi.org/10.1016/j.proeng.2013.08.046.
Wang K, Jiang S, Ma X, Wu Z, Shao H, Zhang W, et al. Information fusion of plume control and personnel escape during the emergency rescue of external-caused fire in a coal mine. Process Saf Environ Prot. 2016;103:46–59. https://doi.org/10.1016/j.psep.2016.06.026.
Lang L, Fu-bao Z. A comprehensive hazard evaluation system for spontaneous combustion of coal in underground mining. Int J Coal Geol. 2010;82(1):27–36. https://doi.org/10.1016/j.coal.2010.01.014.
Wu J-j, Liu X-c. Risk assessment of underground coal fire development at regional scale. Int J Coal Geol. 2011;86(1):87–94. https://doi.org/10.1016/j.coal.2010.12.007.
Taraba B, Pavelek Z. Investigation of the spontaneous combustion susceptibility of coal using the pulse flow calorimetric method: 25 years of experience. Fuel. 2014;125:101–5. https://doi.org/10.1016/j.fuel.2014.02.024.
Xia T, Zhou F, Gao F, Kang J, Liu J, Wang J. Simulation of coal self-heating processes in underground methane-rich coal seams. Int J Coal Geol. 2015;141–142:1–12. https://doi.org/10.1016/j.coal.2015.02.007.
Zhang J, Ren T, Liang Y, Wang Z. A review on numerical solutions to self-heating of coal stockpile: mechanism, theoretical basis, and variable study. Fuel. 2016;182:80–109. https://doi.org/10.1016/j.fuel.2016.05.087.
Qi X, Xue H, Xin H, Wei C. Reaction pathways of hydroxyl groups during coal spontaneous combustion. Can J Chem. 2016;94:1–7. https://doi.org/10.1139/cjc-2015-0605.
Hu E, Zhu C, Rogers K, Han X, Wang J, Zhao J, et al. Coal pyrolysis and its mechanism in indirectly heated fixed-bed with metallic heating plate enhancement. Fuel. 2016;185:656–62. https://doi.org/10.1016/j.fuel.2016.07.115.
Zhang M, Qin T. Development of a new material for mine fire control. Combust Sci Technol. 2014. https://doi.org/10.1080/00102202.2014.890600.
Li M, Wang D, Shan H. Risk assessment of mine ignition sources using fuzzy Bayesian network. Process Saf Environ Prot. 2019;125:297–306. https://doi.org/10.1016/j.psep.2019.03.029.
Guo Q, Ren W, Zhu J, Shi J. Study on the composition and structure of foamed gel for fire prevention and extinguishing in coal mines. Process Saf Environ Prot. 2019;128:176–83. https://doi.org/10.1016/j.psep.2019.06.001.
Chundong S, Hantao L, Li C, Xinhe G. Inert technology application for fire treatment in dead end referred to high gassy mine. Proc Eng. 2011;26:712–6. https://doi.org/10.1016/j.proeng.2011.11.2227.
Wu Z, Hu S, Jiang S, He X, Shao H, Wang K, et al. Experimental study on prevention and control of coal spontaneous combustion with heat control inhibitor. J Loss Prev Process Ind. 2018;56:272–7. https://doi.org/10.1016/j.jlp.2018.09.012.
Qin B, Lu Y, Li Y, Wang D. Aqueous three-phase foam supported by fly ash for coal spontaneous combustion prevention and control. Adv Powder Technol. 2014;25(5):1527–33. https://doi.org/10.1016/j.apt.2014.04.010.
Jia BS, Yin B, Pi ZK, Wen HY. Applying plugging technology to fire prevention of mining without coal pillar. Adv Mater Res. 2012;524–527:543–7. https://doi.org/10.4028/www.scientific.net/AMR.524-527.543.
Zheng X, Zhang D, Wen H. Design and performance of a novel foaming device for plugging air leakage in underground coal mines. Powder Technol. 2019;344:842–8. https://doi.org/10.1016/j.powtec.2018.12.064.
Qin BT, Lu Y. Experimental research on inorganic solidified foam for sealing air leakage in coal mines. Int J Min Sci Technol. 2013;23(1):151–5. https://doi.org/10.1016/j.ijmst.2013.03.006.
Wu J, Yan H, Wang J, Wu Y, Zhou C. Flame retardant polyurethane elastomer nanocomposite applied to coal mines as air-leak sealant. J Appl Polym Sci. 2013. https://doi.org/10.1002/app.38946.
Liu PS, Chen GF. Chapter seven—producing polymer foams. In: Liu PS, Chen GF, editors. Porous materials. Boston: Butterworth-Heinemann; 2014. p. 345–382.
Lu Y. Laboratory study on the rising temperature of spontaneous combustion in coal stockpiles and a paste foam suppression technique. Energy Fuels. 2017. https://doi.org/10.1021/acs.energyfuels.7b00649.
Zhang H, Fang W-Z, Li Y-M, Tao W-Q. Experimental study of the thermal conductivity of polyurethane foams. Appl Therm Eng. 2017;115:528–38. https://doi.org/10.1016/j.applthermaleng.2016.12.057.
Luo F, Wu K, Lu M, Nie S, Li X, Guan X. Thermal degradation and flame retardancy of microencapsulated ammonium polyphosphate in rigid polyurethane foam. J Therm Anal Calorim. 2015;120(2):1327–35. https://doi.org/10.1007/s10973-015-4425-3.
McKenna ST, Hull TR. The fire toxicity of polyurethane foams. Fire Sci Rev. 2016;5(1):3. https://doi.org/10.1186/s40038-016-0012-3.
Yang R, Hu W, Xu L, Song Y, Li J. Synthesis, mechanical properties and fire behaviors of rigid polyurethane foam with a reactive flame retardant containing phosphazene and phosphate. Polym Degrad Stab. 2015;122:102–9. https://doi.org/10.1016/j.polymdegradstab.2015.10.007.
Guo S, Zhang C, Peng H, Wang W, Liu T. Structural characterization, thermal and mechanical properties of polyurethane/CoAl layered double hydroxide nanocomposites prepared via in situ polymerization. Compos Sci Technol. 2011;71(6):791–6. https://doi.org/10.1016/j.compscitech.2010.12.001.
Farhan S, Wang R, Jiang H, Li K. Use of waste rigid polyurethane for making carbon foam with fireproofing and anti-ablation properties. Mater Des. 2016;101:332–9. https://doi.org/10.1016/j.matdes.2016.04.008.
Yuan Y, Yu B, Shi Y, Ma C, Song L, Hu W, et al. Highly efficient catalysts for reducing toxic gases generation change with temperature of rigid polyurethane foam nanocomposites: a comparative investigation. Compos A Appl Sci Manuf. 2018;112:142–54. https://doi.org/10.1016/j.compositesa.2018.05.028.
Doğan F, Özdek N, Selçuki NA, Kaya İ. The synthesis, characterization and effect of molar mass distribution on solid-state degradation kinetics of oligo(orcinol). J Therm Anal Calorim. 2019;138(1):163–73. https://doi.org/10.1007/s10973-019-08211-x.
Ma L, Wang D, Wang Y, Dou G, Xin H. Synchronous thermal analyses and kinetic studies on a caged-wrapping and sustained-release type of composite inhibitor retarding the spontaneous combustion of low-rank coal. Fuel Process Technol. 2017;157:65–75. https://doi.org/10.1016/j.fuproc.2016.11.011.
Wang C, Zhao L, Yuan M, Du Y, Zhu C, Liu Y, et al. Effects of minerals containing sodium, calcium, and iron on oxy-fuel combustion reactivity and kinetics of Zhundong coal via synthetic coal. J Therm Anal Calorim. 2020;139(1):261–71. https://doi.org/10.1007/s10973-019-08439-7.
Maraden A, Stojan P, Matyáš R, Zigmund J. Impact of initial grain temperature on the activation energy and the burning rate of cast double-base propellant. J Therm Anal Calorim. 2019;137(1):185–91. https://doi.org/10.1007/s10973-018-7927-y.
Acknowledgements
This study is funded by the Project of National Natural Science Foundation of China (No. 51604185).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, Y., Guo, P. Experimental investigation on spontaneous combustion of coal affected by exothermic reaction of polyurethane in underground coal mines. J Therm Anal Calorim 147, 337–346 (2022). https://doi.org/10.1007/s10973-020-10312-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-020-10312-x