Abstract
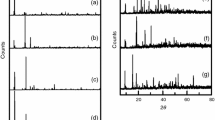
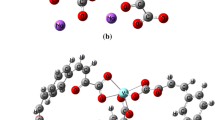
The trivalent lanthanide isonicotinates were synthesized to obtain stoichiometry Lu(IN)3 and Ln(IN)3·2H2O (Ln = Tb to Lu, and Y; IN = isonicotinate). A deep study of the thermal behavior in oxidant (air) and inert (N2) atmospheres was carried out using the following thermoanalytical techniques: simultaneous thermogravimetry and differential scanning calorimetry (TG–DSC), differential scanning calorimetry (DSC) and evolved gas analysis (EGA by TG–DSC–FTIR). From these results, it was possible to determine that dehydration occurs in a single step and the thermal decomposition of the anhydrous compounds occurs in one or two (air), and two or three steps (N2). The final residues of thermal decomposition were Tb4O7 and Ln2O3 (Ln = Dy to Lu, and Y) in air atmosphere, while in N2 atmosphere the mass loss is still being observed up to 1000 °C. The identified gaseous products evolved during the thermal decomposition in dynamic dry air and nitrogen atmospheres were water, CO2, pyridine and CO. From these thermoanalytical data, it was possible to propose a general equation of thermal decomposition of these compounds in the N2 atmosphere. DSC curves of Tb and Ho compounds presented endothermic peaks corresponding to a reversible phase transition, confirmed by powder X-ray diffractometry (XRD), not previously reported. In addition, infrared vibrational spectroscopy (IR) suggests the coordination through carboxylate as bridging bidentate ligand toward the heavy trivalent lanthanides metals. This paper is complementary to our previous study involving the series of light trivalent lanthanides isonicotinates.










Similar content being viewed by others
References
de Sá GF, Malta OL, de Mello Donegá C, Simas a. M, Longo RL, Santa-Cruz PA, et al. Spectroscopic properties and design of highly luminescent lanthanide coordination complexes. Coord Chem Rev. 2000.
Cui Y, Chen B, Qian G. Lanthanide metal-organic frameworks for luminescent sensing and light-emitting applications. Coord Chem Rev. Elsevier BV; 2013;1–11.
Kitchen JA. Lanthanide-based self-assemblies of 2,6-pyridyldicarboxamide ligands: recent advances and applications as next-generation luminescent and magnetic materials. Coord Chem Rev. 2017;340:232–46. https://doi.org/10.1016/j.ccr.2017.01.012.
Abdul’minev IK, Aslanov LA, Porai-Koshits MA, Chupakhina RA. The crystal structure of erbium isonicotinate dihydrate. J Struct Chem. 1973;14:348–50. https://doi.org/10.1007/BF00739482.
Kay J, Moore JW, Glick MD. Structural studies of bridged lanthanide(III) complexes. Diaquotri(nicotinic acid)holmium(III) hexa(isothiocyanato)chromate(III) dihydrate and diaquotris(isonicotinato)lanthanum(III). Inorg Chem. 1972;11:2818–27. https://doi.org/10.1021/ic50117a047.
Chakrabortty M, Ganguli JN, Bora SJ, Das BK. Thermal behaviour of metal (II) isonicotinate tetrahydrates. Indian J Chem. 2010;49:876–81.
Naumova MI, Manicheva EA, Geraski OA, Fedin VP. Synthesis and crystal structures of new lanthanide isonicotinates: coordination polymers and molecular complexes. Russ Chem Bull. 2009;58:1858–65.
Hilder M, Lezhnina M, Junk PC, Kynast UH. Spectroscopic properties of lanthanoid benzene carboxylates in the solid state: part 3. N-heteroaromatic benzoates and 2-furanates. Polyhedron. 2013;52:804–9. https://doi.org/10.1016/j.poly.2012.07.047.
Chen W, Fukuzumi S. Ligand-dependent ultrasonic-assistant self-assemblies and photophysical properties of lanthanide nicotinic/isonicotinic complexes. Inorg Chem. 2009;48:3800–7. https://doi.org/10.1021/ic9000279.
Yin Z, Zhou YL, Zeng MH, Kurmoo M. The concept of mixed organic ligands in metal-organic frameworks: design, tuning and functions. Dalton Trans. 2015;44:5258–75.
Lin XM, Ding YJ, Liang SM, Ge SX, Wei LM, Xie JQ, et al. frameworks constructed from isonicotinate and 2,2′-biphenyldicarboxylate: synthesis, structure and photoluminescence properties. CrystEngComm. 2015;17:3800–8. https://doi.org/10.1039/C5CE00478K.
Ma L, Evans OR, Foxman BM, Lin W. Luminescent lanthanide coordination polymers. Inorg Chem. 1999;38:5837–40. https://doi.org/10.1021/ic990429v.
Nunes WDG, Teixeira JA, do Nascimento ALCS, Caires FJ, Ionashiro EY, Ionashiro M. A comparative study on thermal behavior of solid-state light trivalent lanthanide isonicotinates in dynamic dry air and nitrogen atmospheres. J Therm Anal Calorim. 2016;125:397–405.
Ionashiro M, Graner CAF, Netto JZ. Titulação complexométrica de lantanídeos e ítrio. Eclet Quim. 1983;8:29–32.
Flaschka HA. EDTA titrations. Oxford: Pergamon Press; 1964.
Pastor RC, Gorre LE. Reactive atmosphere processing of oxides (part II). Mater Res Bull. 1987;22:365–71.
Nakamoto K. Infrared and Raman spectra of inorganic and coordination compounds. Part B: applications in coordination, organometallic, and bioinorganic chemistry. 6th ed. Hoboken: Wiley; 2009.
Silverstein RM, Webster FX. spectrometric identification of organic compounds. 6th ed. New York: Wiley; 1998.
Nunes WDG, Teixeira JA, Ekawa B, do Nascimento ALCS, Ionashiro M, Caires FJ. Mn(II), Fe(II), Co(II), Ni(II), Cu(II) and Zn(II) transition metals isonicotinate complexes: thermal behavior in N2 and air atmospheres and spectroscopic characterization. Thermochim Acta. 2018;666:156–65. https://doi.org/10.1016/j.tca.2018.06.010.
Deacon G, Phillips RJ. Relationships between the carbon–oxygen stretching frequencies of carboxylato complexes and the type of carboxylate coordination. Coord Chem Rev. 1980;33:227–50.
Chen W-T, Wen J-W, Luo Z-G, Yao Z-L. Syntheses and characterization of two isonicotinic acid-containing lanthanide complexes with different 1-d polycationic chains. Synth React Inorg Met Nano Metal Chem. 2014;44:1464–8. https://doi.org/10.1080/15533174.2013.809755.
Chen F-Y, Wu W-T, Liu F, Yao L, He S-Y. catena-Poly[[diaqua(isonicotinato-κ 2 O, O′)gadolinium(III)]-di-μ-isonicotinato-κ 4 O: O′]. Acta Crystallogr Sect E Struct. 2007;63:m2151–2. http://scripts.iucr.org/cgi-bin/paper?S160053680703320X.
Wang G, Xu B, Liu H-L, Zheng Q-H. catena-Poly[[diaqua(isonicotinato-κ 2 O, O′)dysprosium(III)]-di-μ-isonicotinato-κ 4 O: O′]. Acta Crystallogr Sect E Struct. 2006;62:m2952–4. http://scripts.iucr.org/cgi-bin/paper?S1600536806041559.
Acknowledgements
The authors thank FAPESP (Proc. 2017/14936-9, 2018/12463-9 and 2018/24378-6), CNPq (Proc. 421469/2016-1) and CAPES foundations (Brazil) for financial support. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (AVI 416422 kb)
Rights and permissions
About this article
Cite this article
Nunes, W.D.G., Teixeira, J.A., do Nascimento, A.L.C.S. et al. Thermal analysis (TG–DSC, DSC–microscopy and EGA) and characterization of heavy trivalent lanthanides and yttrium isonicotinates. J Therm Anal Calorim 138, 309–319 (2019). https://doi.org/10.1007/s10973-019-08112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-019-08112-z