Abstract
The development of hydrogen infrastructure is important because its commercialization will help reduce carbon dioxide emissions significantly. The construction of hydrogen fueling stations will increase the demand for fuel cell vehicles. While the risk associated with various types of fueling stations has been assessed, and appropriate safety regulations have been proposed, there have been few studies on hydrogen fueling stations with on-site dehydrogenation systems that use methylcyclohexane (MCH). This is because such stations are very new. In particular, the thermal hazards associated with such systems must be analyzed because they could lead to equipment damage. The purpose of the present study was to identify the thermal hazards of such systems using various thermal analysis methods. Thermal analyses were performed while assuming spontaneous ignition and oxidation under normal and abnormal conditions, in order to identify the thermal hazards associated with the storage of MCH, toluene, and heat carriers in underground storage tanks as well as their use in the dehydrogenation reactor. In addition, the thermal safety of the tank and the reactor was estimated based on the results of the thermal analyses. It was found that the underground storage tanks for MCH and toluene have a lower thermal risk because the process conditions are mild, and the thermal hazards related to the chemicals are low. Further, in the case of the dehydrogenation reactor, the risk of the spontaneous ignition of the heat carrier is low under quasi-adiabatic conditions and moderate air ventilation, in case the heat carrier leaks from damaged piping and equipment. However, it is important to regularly inspect the reactor to prevent any issues that may arise from an exothermic reaction of the heat carrier.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hydrogen shows significant promise as an energy carrier. An effective use of hydrogen is as a fuel in fuel cell vehicles (FCVs), which emit water and oxygen instead of carbon oxides, nitrogen oxides (NOx), and sulfur oxides (SOx). This characteristic of FCVs should aid the realization of a sustainable society, because such vehicles can help significantly reduce the emissions of the greenhouse gases responsible for climate change. Building hydrogen fueling stations all over the world is essential for the commercialization of FCVs. Hydrogen fueling stations have been constructed in several countries, including the USA, Germany, and Japan. However, safety issues have limited their wide adoption, because these stations store a large amount of hydrogen compressed to 82 MPa in pressurized storage tanks. Hydrogen is inherently hazardous, owing to its explosiveness and embrittlement [1, 2]; these factors significantly increase the risk of accidents. Therefore, improving the safety of hydrogen fueling stations is the key to their wider acceptability.
Hydrogen fueling stations can be categorized into two types: off-site stations, which require the transport of compressed or liquified hydrogen from other places, and on-site stations, which involve the local production of hydrogen by chemical reactions. These stations consist of common components, such as a hydrogen compressor, pressurized storage tanks that store hydrogen at 82 MPa, and dispensers for the delivery of hydrogen to FCVs. The presence of a hydrogen production system is the only difference between on-site and off-site hydrogen fueling stations. Off-site stations are the most common type throughout the world and have had several safety issues, which have been investigated. For example, the leakage and dispersion of compressed or liquified hydrogen have been analyzed both experimentally and using simulations [3, 4]. Further, consequence analysis and risk assessment studies of off-site hydrogen fueling stations have been performed to determine the necessary safety measures [5,6,7,8,9]. In addition, the results of these assessments have led to the development of risk assessment software such as FLACS, HyRAM, and SUSANA [10,11,12,13]. In contrast, the safety issues related to on-site hydrogen fueling stations have not been studied widely, even though doing so could improve the safety of on-site stations significantly. A qualitative risk analysis was performed using HAZID for a hydrogen fueling station with an on-site hydrogen production system involving methylcyclohexane (MCH) [14]. A simulation-based safety investigation of the domino effect was also performed [15]. The results of these studies helped with risk reduction and provided suggestions for safety measures. However, they did not produce concrete safety data for on-site hydrogen production systems, because the studies focused on the holistic risks of the station. Further, because the system studied was at the conceptual design stage, detailed design information such as data related to the equipment size, material, flow rate in pipes, and safety measures was not available. On-site systems carry the risk of temperature and pressure increases, which can accelerate the dehydrogenation of MCH into hydrogen and toluene. However, these thermal hazards have not been studied in detail. These risks can lead to fires or explosions at the stations, causing major injury and property damage, not only to the FCV drivers and passengers using the stations but also to the station maintenance personnel and neighborhood residents. Thermal analysis is a useful technique for identifying and evaluating the thermal hazards of dangerous substances [16, 17], cellulose [18, 19], lithium-ion batteries [20], ionic liquids [21], and reactive monomers [22]. Thus, the purpose of this study was to analyze the thermal hazards of dehydrogenation systems that involve the use of MCH, toluene, and heat carriers, based on thermal analysis equipment. Isothermal and non-isothermal tests were performed for accident scenarios while focusing on the spontaneous ignition of the chemicals involved, in order to elucidate the potential hazards of the system. Based on the analysis results, safety measures for developing safer systems are proposed.
Accident scenarios for on-site hydrogen production systems
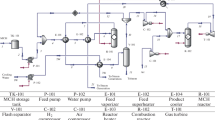
Figure 1 shows the MCH dehydrogenation reaction, while Fig. 2 shows a schematic of an on-site hydrogen production system installed at a hydrogen fueling station. The production system consists of an underground MCH storage tank, dehydrogenation reactor, heat exchanger, gas–liquid separator, hydrogen refinery, and underground toluene storage tank. MCH stored in the tank is transferred to the reactor, where toluene and hydrogen are produced by the dehydrogenation reaction in the presence of a catalyst at ca. 400 °C under 1.0 MPa. This is followed by the separation of the toluene and hydrogen in the separator. The toluene is stored in the tank before being sent to a toluene hydrogenation plant, while the hydrogen is purified and transferred to a pressurized hydrogen tank.
Accident scenarios exist for each component of the system. Three scenarios likely to cause unexpected reactions involving rapidly increasing temperatures and pressures were identified, and experiments were performed based on these scenarios while assuming both normal and abnormal situations.
The first scenario involves the spontaneous ignition of MCH or toluene in the underground storage tanks. Normal tank conditions include ambient temperature and pressure and are similar to those for underground gasoline storage tanks. The consumption of MCH, which produces toluene, requires the storage of toluene over a period of weeks. Oxygen enters the tank when the MCH is delivered, and the toluene is recovered. Introducing inert gases into the tank is not practical. On the other hand, oxygen can oxidize the MCH or toluene, resulting in a thermal distribution because the large size of the tanks results in adiabatic conditions. These events are likely to accelerate the thermal decomposition process and trigger spontaneous ignition in the tank, resulting in a fire. Moreover, if the MCH is stored for long periods, it slowly oxidizes, producing organic peroxides [23], which can decompose violently. Therefore, if a small amount of the organic peroxides derived from MCH were to be produced in the tank, the heat from the exothermic decomposition reaction could lead to a fire involving the MCH.
The second scenario involves the oxidation of MCH, toluene, and the heat carriers within the dehydrogenation reactor. Normal reactor conditions include a temperature of ca. 400 °C and a pressure of less than 1 MPa. Therefore, the oxidation of MCH, toluene, and the heat carriers should not occur. However, MCH containing dissolved oxygen or the oxygen introduced into the system owing to material corrosion or equipment failure can result in oxidation.
The third scenario also involves oxidation but that of the heat carriers in and around the heat exchanger. When passing through a pipe, the heat carriers can leak because of corrosion and erosion, soaking into the adiabatic materials under atmospheric conditions. This situation could inhibit heat release and promote heat accumulation, accelerating the oxidation of the heat carriers and causing spontaneous ignition.
Experimental
The MCH and toluene samples used in this study were obtained from Wako Pure Chemical Industries, Ltd. Two industrial heat carriers, heat carrier_T and heat carrier_N, were also used.
The experimental conditions used were based on the three above-mentioned scenarios. With respect to the first scenario, isothermal tests were conducted using a thermal activity monitor (TAM IV/Q200, TA Instruments). For the TAM IV measurements, ca. 550 mg of MCH or toluene was loaded into a sealed SUS ampoule with a glass inner vessel in air, and the vessel was stored at 30 °C for a week. For the abnormal situation, the thermal degradation products of MCH in air were synthesized; these are referred to as MCH-air. The synthesis conditions involved the storage of MCH at 100 °C in an air atmosphere in a pressurized vessel at 0.5 MPa. After the measurements, the MCH-air compounds were condensed in an N2 atmosphere for 31 h for purification. Non-isothermal screening tests were performed using differential scanning calorimetry (DSC) (Q200, TA Instruments) to evaluate the thermal hazards of MCH-air. During the DSC measurements, ca. 4 mg of MCH and MCH-air was loaded into a SUS303 cell, which was sealed in an Ar atmosphere and heated from 50 to 300 °C at a heating rate of 10 K min−1.
For the second scenario, non-isothermal tests were performed using DSC (HP DSC 827e, Mettler Toledo, and Q200, TA Instruments). During the DSC measurements, ca. 2 mg of MCH, toluene, and the heat carriers was loaded into a SUS303 cell, which was sealed either in air or in an Ar atmosphere and then heated from 30 to 400 °C at a heating rate of 5 or 10 K min−1. The tests did not compare the thermal behaviors of the different samples, as the purpose of the tests was to elucidate the thermal behavior of each sample using screening methods. Therefore, the use of different experimental conditions was not an issue.
For the third scenario, non-isothermal tests were performed using thermogravimetry (TG) (STA2500, Netzsch) measurements. For the measurements, ca. 3–4 mg of the heat carriers was loaded into an alumina cell, which was heated from 30 to 500 °C at 5 K min−1 in air or a He atmosphere. In addition, non-isothermal tests were performed using high-sensitivity heat flux calorimetry (C80, Setaram). Approximately 300 mg of the heat carriers was loaded into a sealed SUS304 vessel with an inner glass vessel in an air atmosphere at 0.1 MPa. The samples were heated from the ambient temperature to 300 °C at a heating rate of 1 K min−1. After the first measurement, the vessel lid was opened, and the vessel was ventilated to increase the amount of oxygen in it. Then, the MCH was reheated under the same conditions, in order to analyze the exothermic reaction related to oxidation. This procedure was performed twice. A chemical composition analysis was performed using Raman spectroscopy (RamanRxn2, Kaiser Optical Systems, Inc.), in order to investigate the causes of oxidation.
Results and discussion
Figure 3 shows the thermal behaviors of MCH and toluene as determined from the isothermal tests performed using TAM IV. Exothermic and endothermic reactions were not observed under the test conditions. The dehydrogenation system is generally operated daily to supply hydrogen to FCVs. Even though the experimental conditions were selected based on generous assumptions, the thermal behavior remained stable for a week. Therefore, it can be concluded that the spontaneous ignition of MCH and toluene in the tanks is highly unlikely under normal conditions.
Figure 4 shows the DSC curves of MCH and MCH-air as determined in Ar. While the thermal behavior of MCH remained unchanged, MCH-air underwent exothermic reactions, with the onset temperature for the first exothermic reaction being ca. 100 °C. Thus, it can be concluded that the thermal hazard is low because the temperature within underground tanks is maintained at ca. 10–20 °C and remains stable throughout the year; exothermic reactions are unlikely to occur under these real-life conditions. In addition, the scenario involving the production of MCH-air and the decomposition of MCH-air is an extreme one and unlikely to occur. Therefore, although the process appears to be dangerous, the risk of a fire in the tank would be very low under both normal and abnormal conditions.
The DSC curves of MCH, toluene, and heat carriers under normal conditions are shown in Fig. 5. Toluene, MCH, heat carrier_T, and heat carrier_N did not undergo exothermic reactions under normal conditions. Therefore, there are no thermal hazards associated with toluene, MCH, heat carrier_N, and heat carrier_T under normal operating conditions.
Figure 6 shows the thermal behaviors of toluene, MCH, heat carrier_T, and heat carrier_N in air. Exothermic reactions were observed in every case, with the onset temperatures being ca. 200, 175, 150, and 150 °C, respectively. The thermal hazard from MCH was very low under these abnormal conditions because the exothermic reaction of MCH was not violent and thus probably will not damage the process equipment and piping. In addition, the dehydrogenation of MCH under abnormal conditions would not produce hydrogen, owing to the presence of the oxidation products of MCH.
Toluene, which is produced by the dehydrogenation of MCH, is recovered to an underground storage tank. Shortly after the reaction, the toluene, which is at an elevated temperature, is cooled and transferred to the tank through a pipe. If a process shutdown occurs owing to a blackout or equipment failure, the toluene stays in the pipes. In such a case, an exothermic reaction may occur under the abnormal conditions. This may prevent the ready release of the heat of the reaction. If this were to occur repeatedly, the resulting thermal stress could damage the pipes at various positions over time, causing fatigue cracks that could allow toluene to leak from the damaged areas. The thermal hazards of toluene are lower than those related to other dangerous substances. However, adequate safety measures, such as periodic inspections, must be taken for the early and timely identification of the hazards.
Although heat carriers are oxidized when air is present in the heat exchange system, they usually do not pose any hazard. However, this can change if a heat carrier leaks from a pipe in the heat exchanger, causing heat accumulation owing to the oxidation of the heat carrier that has seeped into the adiabatic material swaddled around the piping. Spontaneous ignition could occur eventually, because the leakage of the heat carriers from the pipes is difficult to detect through simple inspections. Therefore, investigating the thermal hazards associated with the heat carriers in detail is essential, since DSC is merely a screening tool. Thus, in this study, we also performed TG measurements for analyzing the thermal hazards related to the heat carriers.
Figure 7 shows the TG and differential thermal analysis (DTA) curves of heat carrier_T and heat carrier_N in air and a He atmosphere, while Table 1 lists the onset and end temperatures for mass loss for each heat carrier. The TG curves indicate that the onset and end temperatures in air are higher than those obtained under He. The DTA curves in air indicate that each heat carrier underwent exothermic reactions, while the DTA curves in a He atmosphere are indicative of endothermic reactions. These results suggest that a heat carrier leaking from a damaged pipe does not evaporate readily under atmospheric conditions, in contrast to the case under inert conditions, and that it decomposes through an exothermic reaction. Therefore, if the heat carriers were to leak continuously, their thermal decomposition and heat accumulation would occur interdependently, and spontaneous ignition would likely occur.
The results of the TG tests raised the question as to how many times the heat carriers can leak and get oxidized by air. To determine the answer, repeated thermal analyses were performed using C80.
The thermal behaviors of pure heat carriers as well as the reheated heat carriers are shown in Fig. 8. The exothermic onset temperatures of heat carrier_T and heat carrier_N were ca. 180 °C and ca. 170 °C, respectively. These results are in keeping with those of the DSC and TG measurements, namely that the thermal behavior of heat carrier_N is similar to that of heat carrier_T. The usefulness of the thermal analysis instruments used in this study is limited to closed vessels containing very small amounts of oxygen and may underestimate the extent of oxidation in the absence of oxygen. Therefore, repeated measurements were performed using C80. The thermal behaviors of the heat carriers heated two and three times indicated that they underwent exothermic reactions, showing onset temperatures of ca. 160 and 170 °C, respectively. This suggests that the air continues to oxidize the heat carriers when a large amount of oxygen is present in it. The heated heat carriers turned from colorless to clear yellow. Figure 9 shows the Raman shifts of heat carrier_N and the heated heat carrier_N; however, the reason for the color change of the heat carriers could not be determined. These measurement results indicate that spontaneous ignition can occur under quasi-adiabatic conditions and moderate air ventilation when a heat carrier leaks from damaged piping and equipment. The detection of such leaks by inspection may be difficult; however, the change in the color of the oxidized heat carrier to yellow can aid in leak detection during inspections.
Conclusions
In this study, we attempted to estimate the thermal hazards related to the MCH dehydrogenation system using TAM IV, DSC, TG, and C80 measurements. The results obtained can be summarized as follows:
-
1.
Under normal operating conditions, the thermal hazards of MCH, toluene, and the heat carriers are very low, and the dehydrogenation system can be operated safely.
-
2.
Under abnormal operating conditions, thermal hazards exist, and minor incidents can occur. However, fatal accidents are less likely. The leakage of the heat carriers can be likely to cause the spontaneous ignition of the heat carrier system. Therefore, regular and thorough inspections of the system are essential.
-
3.
Process safety assessments of the dehydrogenation system must be included at the design stage based on the thermal hazard data obtained in this study.
References
Rigas F, Amyotte P. Hydrogen safety. Boca Raton: CRC Press; 2013.
Crowl DA, Jo YD. The hazards and risks of hydrogen. J Loss Prev Process Ind. 2007;20:158–64.
Hall JE, Hooker P, Willoughby D. Ignited release of liquid hydrogen: safety consideration of thermal and overpressure effects. Int J Hydrogen Energy. 2014;39:20547–53.
Daubech J, Hebrard J, Jallais S, Vyazmina E, Jamois D, Verbecke F. Un-ignited and ignited high pressure hydrogen release: concentration—turbulence mapping and overpressure effects. J Loss Prev Process Ind. 2015;36:439–46.
Matthijsen AJCM, Kooi ES. Safety distances for hydrogen filling station. J Loss Prev Process Ind. 2006;19:719–23.
Kikukawa S, Mitsuhashi H, Miyake A. Risk assessment for liquid hydrogen fueling stations. Int J Hydrogen Energy. 2009;34:1135–41.
Kikukawa S. Consequence analysis and safety verification of hydrogen fueling stations using CDF simulation. Int J Hydrogen Energy. 2008;33:1425–34.
Kim E, Lee K, Kim J, Lee Y, Park J, Moon I. Development of Korean hydrogen fueling station codes through risk analysis. Int J Hydrogen Energy. 2001;36:13122–31.
Sakamoto J, Nakayama J, Nakarai T, Kasai N, Shibutani T, Miyake A. Effect of gasoline pool fire on liquid hydrogen storage tank in hybrid hydrogen–gasoline fueling station. Int J Hydrogen Energy. 2016;41:2096–104.
Middha P, Hansen OR, Storvik IE. Validation of CFD-model for hydrogen dispersion. J Loss Prev Process Ind. 2009;22:1034–8.
Hansen OR, Middha P. CFD-based risk assessment for hydrogen application. Process Saf Prog. 2008;27:29–34.
Baraldi D, Melideo D, Kotchourko A, Ren K, Yanez J, Jedicke O, Giannissi SG, Tolias IC, Venetsanos AG, Keenan J, Makarov D, Molkov V, Slater S, Verbecke F, Duclos A. Development of a model evaluation protocol for CFD analysis of hydrogen safety issues the SUSANA project. Int J Hydrogen Energy. 2017;42:7633–43.
Sandia National Laboratories. Hydrogen risk assessment model (HyRAM). http://energy.sandia.gov/transportation-energy/hydrogen/quantitative-risk-assessment/hydrogen-risk-assessment-model-hyram/.
Nakayama J, Sakamoto J, Kasai N, Shibutani T, Miyake A. Preliminary hazard identification for qualitative risk assessment on a hybrid gasoline-hydrogen fueling station with an on-site hydrogen production system using organic chemical hydride. Int J Hydrogen Energy. 2016;41:7518–25.
Nakayama J, Misono H, Sakamoto J, Kasai N, Shibutani T, Miyake A. Simulation-based safety investigation of a hydrogen fueling station with an on-site hydrogen production system involving methylcyclohexane. Int J Hydrogen Energy. 2017;42:10636–44.
Mathieu D, Alaime T, Beaufrez J. From theoretical energy barriers to decomposition temperatures of organic peroxides. J Therm Anal Calorim. 2017;129:323–37.
Popławski D, Hoffmann J, Hoffmann K. Effect of carbonate minerals on the thermal stability of fertilisers containing ammonium nitrate. J Therm Anal Calorim. 2016;124:1561–74.
Nakayama J, Miyake A. Catalytic effect of copper(II) oxide on oxidation of cellulosic biomass. J Therm Anal Calorim. 2012;110:321–7.
Nakayama J, Miyake A. Thermal and evolved gas analyses of the oxidation of a cellulose/copper(II) oxide mixture. J Therm Anal Calorim. 2013;113:1403–8.
Wang Q, Zhao X, Ye J, Sun Q, Ping P, Sun J. Thermal response of lithium-ion battery during charging and discharging under adiabatic conditions. J Therm Anal Calorim. 2016;124:417–28.
Yamamoto Y, Miyake A. Influence of a mixed solvent containing ionic liquids on the thermal hazards of the cellulose dissolution process. J Therm Anal Calorim. 2017;127:743–8.
Fujita M, Iizuka Y, Miyake A. Thermal and kinetic analyses Michael addition reaction of acrylic acid. J Therm Anal Calorim. 2017;128:1227–33.
Syroezhko AM, Begak OY, Proskuryakov VA. Mechanism of methylcyclohexane ozonolysis. Russ J Appl Chem. 2003;76(5):785–90.
Acknowledgements
This work was supported by the Council for Science, Technology and Innovation (CSTI) through its Cross-ministerial Strategic Innovation Promotion Program (SIP), “Energy Carrier” [Funding agency: Japan Science and Technology Agency (JST)].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Nakayama, J., Aoki, H., Homma, T. et al. Thermal hazard analysis of a dehydrogenation system involving methylcyclohexane and toluene. J Therm Anal Calorim 133, 805–812 (2018). https://doi.org/10.1007/s10973-018-6971-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-018-6971-y