Abstract
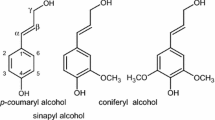
Soda lignins from three non-wood species, namely bamboo (Bambusa bambos), giant cane (Arundo donax) and pearl millet (Pennisetum glaucum) were studied for their thermolytic degradation behaviour. TG characterization of the soda lignins were done under nitrogen atmosphere at two heating rates of 10 and 100 °C min−1. After the initial moisture removal, two distinct thermal degradation events were observed. However, thermal degradation at 100 °C min−1 shifted the onset of thermal degradations to higher temperatures. Both the thermal degradation of all the three lignins was found to be exothermic except for first thermal degradation of pearl millet lignin during thermal degradation at 10 °C min−1 which was almost athermic. Batch thermal degradation runs on the three lignins were carried out at different temperatures for varying lengths of time and the FTIR spectra of the thermal degradation residues (TDRs) were recorded. At 200 °C, the marginal mass loss of less than 10% is attributed to moisture loss. At lower temperature of 250 °C, the intramolecular hydrogen bonding through hydroxyl group in the original lignin was broken. Partial to complete decarboxylation also took place. The aromatic structure of the lignin largely remained intact albeit with rearrangements. Short thermal degradation for 1 h at 250 °C yielded 75–80% residue for the three lignins.







Similar content being viewed by others
References
Ghatak HR, Kundu PP, Kumar S. Thermochemical comparison of lignin separated by electrolysis and acid precipitation from soda black liquor of agricultural residues. Thermochim Acta. 2010;502:85–9.
Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–46.
Ragauskas AJ, Beckham GT, Biddy MJ, Chandra R, Chen F, Davis MF, Davison BH, Dixon RA, Gilna P, Keller M, Langan P, Naskar AK, Saddler JN, Tschaplinski TJ, Tuskan GA, Wyman CE. Lignin valorization: improving lignin processing in the biorefinery. Science. 2014. https://doi.org/10.1126/science.1246843.
Ghatak HR. Spectroscopic comparison of lignin separated by electrolysis and acid precipitation of wheat straw soda black liquor. Ind Crops Prod. 2008;28:206–12.
Garcia A, Toledano A, Serrano L, Egues I, Gonzalez M, Marin F, Labidi J. Characterization of lignins obtained by selective precipitation. Sep. Purific. Technol. 2009;68:193–8.
Fernandez-Rodriguez J, Gordobil O, Robles E, Gonzalez-Alriols M, Labidi J. Lignin valorization from side-streams produced during agricultural waste pulping and total chlorine free bleaching. J. Cleaner Prod. 2016. https://doi.org/10.1016/j.jclepro.2016.10.198.
Wu C, Wang Z, Dupont V, Huang J, Williams PT. Nickel-catalysed pyrolysis/gasification of biomass components. J Anal Appl Pyrol. 2013;99:143–8.
Gooty AT, Li D, Berruti F, Briens C. Kraft-lignin pyrolysis and fractional condensation of its bio-oil vapors. J Anal Appl Pyrol. 2014;106:33–40.
Kleinert M, Barth T. Phenols from lignin. Chem Eng Technol. 2008;31:736–45.
Yang H, Yan R, Chen H, Lee DH, Zheng C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel. 2007;86:1781–8.
Chen T, Li L, Zhao R, Wu J. Pyrolysis kinetic analysis of the three pseudocomponents of biomass–cellulose, hemicellulose and lignin. J Therm Anal Calorim. 2017;128:1825–32.
Zhang J, Jiang X, Ye X, Chen L, Lu Q, Wang X, Dong C. Pyrolysis mechanism of a β-O-4 type lignin dimer model compound. J Therm Anal Calorim. 2016;123:501–10.
Ferdous D, Dalai AK, Bej SK, Thring RW. Production of H2 and medium heating value gas via steam gasification of lignins in fixed-bed reactors. Can J Chem Eng. 2001;79:913–22.
Ferdous D, Dalai AK, Bej SK, Thring RW. Pyrolysis of Lignins: experimental and kinetics studies. En. Fuels. 2002;16:1405–12.
Fushimi C, Araki K, Yamaguchi Y, Tsutsumi A. Effect of heating rate on steam gasification of biomass. 1. reactivity of char. Ind Eng Chem Res. 2003;42:3922–8.
Guan Q, Mao T, Zhang Q, Miao R, Ning P, Gu J, Tian S, Chen Q, Chai X. Catalytic gasification of lignin with Ni/Al2O3–SiO2 in sub/supercritical water. J. Supercritical Fluids. 2014;95:413–21.
Jiang G, Nowakowski DJ, Bridgwater AV. A systematic study of the kinetics of lignin pyrolysis. Thermochim Acta. 2010;498:61–6.
Singh R, Prakash A, Dhiman SK, Balagurumurthy B, Arora AK, Puri SK, Bhaskar T. Hydrothermal conversion of lignin to substituted phenols and aromatic Ethers. Bioresource Technol. 2014;165:319–22.
Farag S, Mudraboyina BP, Jessop PG, Chaouki J. Impact of the heating mechanism on the yield and composition of bio-oil from pyrolysis of kraft lignin. Biomass Bioen. 2016;95:344–53.
Jie-wang Y, Gui-zhen F, Chun-de J. Hydrogenation of alkali lignin catalyzed by Pd/C. APCBEE Procedia. 2012;3:53–9.
Mattsson C, Andersson S, Belkheiri T, Amand L, Olausson L, Vamling L, Theliander H. Using 2D NMR to characterize the structure of the low and high molecular mass fractions of bio-oil obtained from LignoBoost™ kraft lignin depolymerized in subcritical water. Biomass Bioen. 2016;95:364–77.
Hatakeyama H, Tsujimoto Y, Zarubin MJ, Krutov SM, Hatakeyama T. Thermal decomposition and glass transition of industrial hydrolysis lignin. J Therm Anal Calorim. 2010;101:289–95.
Ye X, Lu Q, Jiang X, Wang X, Hu B, Li W, Dong C. Interaction characteristics and mechanism in the fast co-pyrolysis of cellulose and lignin model compounds. J Therm Anal Calorim. 2017;130:975–84.
Jiang G, Nowakowski DJ, Bridgwater AV. Effect of the temperature on the composition of lignin pyrolysis products. Energy Fuels. 2010;24:4470–5.
Bai X, Kim KH, Brown RC, Dalluge E, Hutchinson C, Lee YJ, Dalluge D. Formation of phenolic oligomers during fast pyrolysis of lignin. Fuel. 2014;128:170–9.
Mullen CA, Boateng AA. Catalytic pyrolysis-GC/MS of lignin from several sources. Fuel Proces. Technol. 2010;91:1446–58.
Li X, Su L, Wang Y, Yu Y, Wang C, Li X, Wang Z. Catalytic fast pyrolysis of Kraft lignin with HZSM-5 zeolite for producing aromatic hydrocarbons. Front. Environ. Sci. Eng. 2012;6:295–303.
Shen DK, Gu S, Luo KH, Wang SR, Fang MX. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresource Technol. 2010;101:6136–46.
El Mansouri N, Yuan Q, Huang F. Characterization of lignins for use in phenol formaldehyde and epoxy resins. BioRes. 2011;6:2647–62.
Sharma RK, Wooten JB, Baliga VL, Lin X, Chan WG, Hajaligol MR. Characterization of chars from pyrolysis of lignin. Fuel. 2004;83:1469–82.
Watkins D, Nuruddin M, Hosur M, Tcherbi-Narteh A, Jeelani S. Extraction and characterization of lignin from different biomass resources. J. Mater. Res. Technol. 2015;4:26–32.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, I.A.K., Ghatak, H.R. Low-temperature thermal degradation behaviour of non-wood soda lignins and spectroscopic analysis of residues. J Therm Anal Calorim 132, 407–423 (2018). https://doi.org/10.1007/s10973-017-6912-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6912-1