Abstract
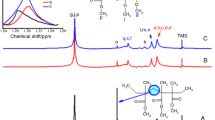
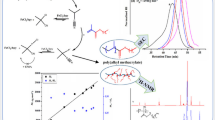
Well-defined copolymers of ethylene and butyl methacrylate (BMA) (poly(ethylene-co-BMA)) with narrow molecular weight distribution were synthesized via dual concurrent atom transfer radical polymerization (ATRP) and reversible addition–fragmentation chain transfer (RAFT) polymerization using 4-cyano-4-(phenylcarbonothioylthio)pentanoic acid as an alkyl pseudo-halide initiator, ascorbic acid/FeCl3/2,2′ bipyridine as the ATRP catalyst system and ethyl-2-bromo-isobutyrate as co-initiator in N, N′-dimethyl formamide at 100 °C. From size exclusion chromatography result, controlled molecular weight with narrow molecular weight distributions of poly(ethylene-co-BMA) was obtained in every case. The experimental results indicated that dual concurrent ATRP–RAFT polymerization is more successful over free radical or RAFT polymerization in terms of controlling molecular weight and polydispersity. X-ray diffraction analysis demonstrated the amorphous behaviour of poly(ethylene-co-BMA) while thermogravimetric analysis reveals that the main decomposition of poly(ethylene-co-BMA) occurs in the range 300–420 °C. The multivariate nonlinear regression analysis was performed to establish the mechanism and kinetic model and found that n-dimensional Avrami–Erofeev (An) mechanism was responsible for the decomposition of the synthesized poly(ethylene-co-BMA). The apparent activation energy (E a) of decomposition of the synthesized copolymer was found to be 211.88 kJ mol−1.








Similar content being viewed by others
References
Chung TC. Synthesis of functional polyolefin copolymers with graft and block structures. Prog Polym Sci. 2002;27:39–85.
Passaglia E, Coiai S, Cicogna F, Ciardelli F. Some recent advances in polyolefin functionalization. Polym Int. 2014;63:12–21.
Hong SC, Jia S, Teodorescu M, Kowalewski T, Matyjaszewski K, Gottfried AC, Brookhart M. Polyolefin graft copolymers via living polymerization techniques: preparation of poly (n-butyl acrylate)-graft-polyethylene through the combination of Pd-mediated living olefin polymerization and atom transfer radical polymerization. J Polym Sci Part A Polym Chem. 2002;40:2736–49.
Sugimoto R, Kaneko H, Saito J, Kawahara N, Matsuo S, Matsugi T. Controlled radical polymerization with polyolefin macroinitiator: a convenient and versatile approach to polyolefin-based block and graft copolymers. Polym Bull. 2014;71:1421–31.
Zhang K, Ye Z, Subramanian R. Synthesis of block copolymers of ethylene with styrene and n-butyl acrylate via a tandem strategy combining ethylene “Living” polymerization catalyzed by a functionalized Pd-diiminecatalyst with atom transfer radical polymerization. Macromolecules. 2008;41:640–9.
Yanjarappa MJ, Shrivram S. Recent developments in the synthesis of functional poly(olefin)s. Prog Polym Sci. 2002;27:1347–98.
Moad G. The synthesis of polyolefin graft copolymers by reactive extrusion. Prog Polym Sci. 1999;24:81–142.
Franssen NMG, Reek JNH, de Bruin B. Synthesis of functional “polyolefins”: state of the art and remaining challenges. Chem Soc Rev. 2013;42:5809–32.
Kato M, Kamigaito M, Sawamoto M, Higashimuras T. Polymerization of methyl Methacrylate with the carbon tetrachloride/dichlorotris- (triphenylphosphine)ruthedum(II)/methylaluminumbis(2,6-di-tert-butylphenoxide) initiating system: possibility of living radical polymerization. Macromolecules. 1996;28:1721–3.
Nicolay R, Kwak Y, Matyjaszewski K. Dibromotrithiocarbonate iniferter for concurrent ATRP and RAFT polymerization. Effect of monomer, catalyst, and chain transfer agent structure on the polymerization mechanism. Macromolecules. 2008;41:4585–96.
Teodorescu M, Matyjaszewski K. Atom transfer radical polymerization of (meth)acrylamides. Macromolecules. 1999;32:4826–31.
Mori H, Muller AHE. New polymeric architectures with (meth)acrylic acid segments. Prog Polym Sci. 2003;28:1403–39.
Elsen AM, Nicolay R, Matyjaszewski K. Dual concurrent ATRP/RAFT of methyl acrylate co-initiated by alkyl halides. Macromolecules. 2011;44:1752–4.
Zhao Y, Perrier S. Synthesis of poly (methyl acrylate) grafted onto silica particles by z-supported RAFT Polymerization. Radic Polym Kinet Mech. 2007;248:94–103.
Hornung CH, Nguyen X, Kyi S, Chiefari J, Saubern S. Synthesis of RAFT block copolymers in a multi-stage continuous flow process inside a tubular reactor. Aust J Chem. 2013;66:192–8.
Kwak Y, Yamamura Y, Matyjaszewski K. ATRP of styrene and methyl methacrylate with less efficient catalysts and with alkyl pseudohalides as initiators/chain transfer agents. Macromol Chem Phys. 2010;211:493–500.
Pan J, Miao J, Zhang L, Si Z, Zhang C, Cheng Z, Zhu X. Iron-mediated (dual) concurrent ATRP–RAFT polymerization of water-soluble poly (ethylene glycol) monomethyl ether methacrylate. Polym Chem. 2013;4:5664–70.
Peterson JD, Vyazovkin S, Wight CA. Kinetics of the thermal and thermo-oxidative degradation of polystyrene, polyethylene and poly (propylene). Macromol Chem Phys. 2001;202:775–84.
Roy PK, Surekha P, Rajagopa C, Choudhary V. Thermal degradation studies of LDPE containing cobalt stearate as pro-oxidant. Express Polym Lett. 2007;1:208–16.
Kayacan I, Dogan OM. Pyrolysis of low and high-density polyethylene. Part I: non-isothermal pyrolysis kinetics. Energy Sources Part A Recover Util Environ Eff. 2008;30:385–91.
Razuvaev GA, Troitskii BB, Chochlova LV, Dubova ZB. Thermal degradation of ethylene-vinyl acetate copolymer. Polym Lett Ed. 1973;11:521–3.
Koleva D, Atanassov A. Non-isothermal kinetics of degradation of ultra-high molecular mass polyethene composite materials: part I. Composite materials with fiber monocrystals. J Therm Anal Calorim. 2008;91:213–8.
Lee YJ, Litzinger TA. Thermal decomposition of BAMO/AMMO with and without TiO2. Thermochim Acta. 2002;384:121–35.
Assanvo EF, Konwar D, Baruah SD. Thermal behavior of Ricinodendronheudelotii oil polymer. J Therm Anal Calorim. 2015;119:1995–2003.
Vincent BJ, Natarajan B. Kinetics of thermal degradation of water borne polyurethane dispersion containing polycaprolactone with either isophoronediisocyanate or metatetramethyl xylene diisocyanate. J Therm Anal Calorim. 2015;119:1373–9.
Salehia M, Clemens F, Graule T, Grobéty B. Kinetic analysis of the polymer burnout in ceramic thermoplastic processing of the YSZ thin electrolyte structures using model free method. Appl Energy. 2012;95:147–55.
Kahrizsangi RE, Abbasi MH. Evaluation of reliability of Coats-Redfern method for kinetic analysis of non-isothermal TGA. Trans Nonferrous Met Soc China. 2008;18:217–21.
Manafi P, Ghasemi I, Karrabi M, Azizi H, Manafi MR, Ehsaninamin P. Thermal stability and thermal degradation kinetics (model-free kinetics) of nanocomposites based on poly (lactic acid)/graphene: the influence of functionalization. Polym Bull. 2015;72:1095–112.
FotsoTalla AS, Erchiqui F, Godard F, Kocaefe D. An evaluation of the thermal degradation kinetics of novel melt processed PET–hemp fibre composites. J Therm Anal Calorim. 2016;126:1387–96.
Baruah U, Saikia M, Assanvo EF, Borphukan S, Phukan L, Gautam A, Baruah SD. Synthesis and thermal analysis of poly (methyl methacrylate) oligomer functionalized polyethylene block copolymer. Polym Bull. 2017;74:2137–58.
Das T, Baruah BP, Saikia BK. Thermal behaviour of low-rank Indian coal fines agglomerated with an organic binder. J Therm Anal Calorim. 2016;126:435–46.
Yaghini N, Iedema PD. Molecular weight and branching distribution modeling in radical polymerization with transfer to polymer and scission under gel conditions and allowing for multiradicals. Macromolecules. 2014;47:4851–63.
Bakhshi H, Zohuriaan-Mehr MJ, Bouhendi H, Kabiri K. Spectral and chemical determination of copolymer composition of poly (butyl acrylate-co-glycidyl methacrylate) from emulsion polymerization. Polym Test. 2009;28:730–6.
Balamurugan A, Kannan S, Selvaraj V, Rajeswari S. Development and spectral characterization of poly (methyl methacrylate)/hydroxyapatite composite for biomedical applications. Trends Biomater Artif Organs. 2004;18:41–5.
Yazdimamaghani M, Pourvala T, Motamedi E, Fathi B, Vashaee D, Tayebi L. Synthesis and characterization of encapsulated nanosilica particles with an acrylic copolymer by in situ emulsion polymerization using thermoresponsive nonionic surfactant. Materials (Basel). 2013;6:3727–41.
Todorovic ZS, Nikolic LB, Nikolic VD, Vukovic ZM. Cross-lnked macroporous polymers and copolymers, their synthesis and characterization. Adv Technol. 2012;1:11–9.
Diamanti SJ, Khanna V, Hotta A, Coffin RC, Yamakawa D, Kramer EJ, Fredrickson GH, Bazan GC. Tapered block copolymers containing ethylene and a functionalized comonomer. Macromolecules. 2006;39:3270–4.
Gaur U, Underlich B. The glass transition temperature of polyethylene. Macromolecules. 1980;13:445–6.
Penzel E, Rieger J, Schneider HA. The glass transition temperature of random copolymers: 1. Experimental data and the Gordon-Taylor equation. Polymer. 1997;38:325–37.
Czech Z, Pełech R, Zych K, Swiderska J. Thermal degradation of copolymers based on selected alkyl methacrylates. J Therm Anal Calorim. 2012;109:573–6.
Jankovic B, Mentus S, Jankovic M. A kinetic study of the thermal decomposition process of potassium metabisulfite: estimation of distributed reactivity model. J Phys Chem Solids. 2008;69:1923–33.
Acknowledgements
The authors wish to thank Dr. D. Ramaiah, Director, CSIR-NEIST, Jorhat for permission to publish the results. The authors are very much thankful to the CSIR, New Delhi, for funding the Network Project CSC-0206. Authors express special thanks to Dr. Binoy K. Saikia and Mr. Tonkeswar Das for their assistance in thermal analysis.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Saikia, M., Borphukan, S., Baruah, U. et al. Poly(ethylene-co-BMA) via dual concurrent ATRP–RAFT and its thermokinetic study. J Therm Anal Calorim 131, 1517–1526 (2018). https://doi.org/10.1007/s10973-017-6536-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-017-6536-5