Abstract
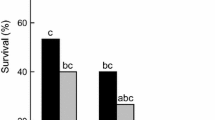
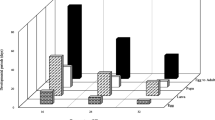
The longevity of Asian citrus psyllid, Diaphorina citri, infected by an entomopathogenic fungus, Isaria fumosorosea, was studied by microcalorimetry. Experimental results of microcalorimetry suggest that in the process of metabolism of D. citri heat or heat flow declined exponentially. The metabolism of D. citri inhibited by increasing temperature, and the longevity of D. citri decreased gradually with respect to temperature. The longevity of D. citri almost reaches 500 h at 25 °C and D. citri died within 130 h at 41 °C under high-temperature exposure. The same phenomenon, decrease in longevity and metabolism inhibition, was observed under the low-temperature exposure. The supercooling point of insects can be derived from a cooling curve plotting the temperature versus time by using microcalorimetry. The heat released due to phase change can be determined by using microcalorimeter. Our results indicated that microcalorimetry can be used to measure the metabolism of herbivore insects.






Similar content being viewed by others
References
Huang CH, Tsai MY, Wang CL. Transmission of citrus Likubin by a psyllid, Diaphorina citri. J Agric Res China. 1984;1(33):65–72.
Grafton-Cardwell EE, Stelinski LL, Stansly PA. Biology and management of Asian citrus psyllid, vector of the huanglongbing pathogens. Annu Rev Entomol. 2013;58:413–32. doi:10.1146/annurev-ento-120811-153542.
Yan H, Zeng J, Zhong G. The push-pull strategy for citrus psyllid control. Pest Manag Sci. 2015;71(7):893–6. doi:10.1002/ps.3915.
Halbert SE. Asian citrus psyllid—a serious potential exotic pest of Florida citrus. http://www.doacsstateflus/~pi/enpp/ento/dcitrihtm (1997).
Hall DG, Rohrig E. Bionomics of Asian citrus psyllid (Hemiptera: Liviidae) associated with orange jasmine hedges in Southeast Central Florida, with special reference to biological control by Tamarixia radiata. J Econ Entomol. 2015;108(3):1198–207. doi:10.1093/jee/tov052.
Qureshi JA, Rogers ME, Hall DG, Stansly PA. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiata (Hymenoptera: Eulophidae) in Florida citrus. J Econ Entomol. 2009;102(1):247–56.
Khan SZ, Arif MJ, Hoddle CD, Hoddle MS. Phenology of Asian citrus psyllid (Hemiptera: Liviidae) and associated parasitoids on two species of Citrus, kinnow mandarin and sweet orange, in Punjab Pakistan. Environ Entomol. 2014;43(5):1145–56. doi:10.1603/EN14093.
Avery PB, Pick DA, Aristizabal LF, Kerrigan J, Powell CA, Rogers ME, et al. Compatibility of Isaria fumosorosea (Hypocreales: Cordycipitaceae) blastospores with agricultural chemicals used for management of the Asian citrus psyllid, diaphorina citri (Hemiptera: Liviidae). Insects. 2013;4(4):694–711. doi:10.3390/insects4040694.
Avery PB, Wekesa VW, Hunter WB, Hall DG, McKenzie CL, Osborne LS, et al. Effects of the fungus Isaria fumosorosea (Hypocreales: Cordycipitaceae) on reduced feeding and mortality of the Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Biocontrol Sci Technol. 2011;21(9):1065–78. doi:10.1080/09583157.2011.596927.
Semenitz E, Tiefenbrunner F. Microcalorimetric studies on determining antibacterial activity of chemotherapeutic agents (author’s transl). Arzneimittelforschung. 1977;27(12):2247–51.
Guan Y, Evans PM, Kemp RB. Specific heat flow rate: an on-line monitor and potential control variable of specific metabolic rate in animal cell culture that combines microcalorimetry with dielectric spectroscopy. Biotechnol Bioeng. 1998;58(5):464–77.
Chen XY, Hu XL, Xia CF, Qin CQ, Liu Y. Antibacterial evaluation of novel organoarsenic compounds by the microcalorimetric method. Biol Trace Elem Res. 2013;153(1–3):382–9. doi:10.1007/s12011-013-9660-5.
Lu TC, Zhang YZ, Huang ZH, Huang J. The effect on the longevity of Leptocybe invasa Fisher & LaSalle (Hymenoptera: Eulophidae) studied by microcalorimetry and traditional methods. J Therm Anal Calorim. 2014;116(1):461–7. doi:10.1007/s10973-013-3572-7.
Henes ST, Johnson A, Toner M, Mamaril K, Kelkar M, Xiao Y, et al. Assessing resting metabolic rate in overweight and obese adolescents with a portable indirect calorimeter: a pilot study for validation and reliability. Nutr Clin Pract. 2015;. doi:10.1177/0884533615603966.
Yatabe T, Kitagawa H, Yamashita K, Hanazaki K, Yokoyama M. Energy expenditure measured using indirect calorimeter after minimally invasive esophagectomy in ventilated postoperative patients. Asia Pac J Clin Nutr. 2014;23(4):555–9. doi:10.6133/apjcn.2014.23.4.20.
Nagano Y, Ode KL. Temperature-independent energy expenditure in early development of the African clawed frog Xenopus laevis. Phys Biol. 2014;11(4):046008. doi:10.1088/1478-3975/11/4/046008.
Leon LR, Dineen S, Blaha MD, Rodriguez-Fernandez M, Clarke DC. Attenuated thermoregulatory, metabolic, and liver acute phase protein response to heat stroke in TNF receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2013;305(12):R1421–32. doi:10.1152/ajpregu.00127.2013.
Burnett CM, Grobe JL. Direct calorimetry identifies deficiencies in respirometry for the determination of resting metabolic rate in C57Bl/6 and FVB mice. Am J Physiol Endocrinol Metab. 2013;305(7):E916–24. doi:10.1152/ajpendo.00387.2013.
Piaggi P, Thearle MS, Bogardus C, Krakoff J. Lower energy expenditure predicts long-term increases in weight and fat mass. J Clin Endocrinol Metab. 2013;98(4):E703–7. doi:10.1210/jc.2012-3529.
Wang H, Zhang YF, Xu LL, Jiang CM. Step rate-determined walking intensity and walking recommendation in Chinese young adults: a cross-sectional study. BMJ Open. 2013;. doi:10.1136/bmjopen-2012-001801.
Frankenfield DC, Coleman A. An evaluation of a handheld indirect calorimeter against a standard calorimeter in obese and nonobese adults. JPEN J Parenter Enter Nutr. 2013;37(5):652–8. doi:10.1177/0148607112473340.
Mori S, Choi J, Devireddy RV, Bischof JC. Calorimetric measurement of water transport and intracellular ice formation during freezing in cell suspensions. Cryobiology. 2012;65(3):242–55. doi:10.1016/j.cryobiol.2012.06.010.
Dhandapani B, Mahadevan S, Mandal AB. Energetics of growth of Aspergillus tamarii in a biological real-time reaction calorimeter. Appl Microbiol Biotechnol. 2012;93(5):1927–36. doi:10.1007/s00253-011-3722-4.
Schuler MM, Sivaprakasam S, Freeland B, Hama A, Hughes KM, Marison IW. Investigation of the potential of biocalorimetry as a process analytical technology (PAT) tool for monitoring and control of Crabtree-negative yeast cultures. Appl Microbiol Biotechnol. 2012;93(2):575–84. doi:10.1007/s00253-011-3507-9.
Baumgartner A, Steurer A, Tiefenbock W, Gabris F, Maringer FJ, Kapsch RP, et al. Re-evaluation of correction factors of a primary standard graphite calorimeter in 60Co gamma ray beams as a basis for the appointment of the BEV absorbed dose rate to water reference value. Radiat Prot Dosim. 2011;145(1):3–12. doi:10.1093/rpd/ncq376.
Alves GR, Diniz AJ, Parra JR. Biology of the Huanglongbing vector Diaphorina citri (Hemiptera: Liviidae) on different host plants. J Econ Entomol. 2014;107(2):691–6.
Kabanova N, Stulova I, Vilu R. Microcalorimetric study of the growth of bacterial colonies of Lactococcus lactis IL1403 in agar gels. Food Microbiol. 2012;29(1):67–79. doi:10.1016/j.fm.2011.08.018.
Wadso I. Isothermal microcalorimetry in applied biology. Thermochim Acta. 2002;394(1–2):305–11. doi:10.1016/S0040-6031(02)00263-0.
Jayaprakasha GK, Rao LJM, Sakariah KK. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J Agric Food Chem. 2002;50(13):3668–72. doi:10.1021/jf025506a.
Jin C, Kong WJ, Luo Y, Wang JB, Wang HT, Li QM, et al. Development and validation of UPLC method for quality control of Curcuma longa Linn.: fast simultaneous quantitation of three curcuminoids. J Pharm Biomed. 2010;53(1):43–9. doi:10.1016/j.jpba.2010.03.021.
Takaisi-Kikuni NB, Kruger D, Gnann W, Wecke J. Microcalorimetric and electron microscopic investigation on the effects of essential oil from Cymbopogon densiflorus on Staphylococcus aureus. Microbios. 1996;88(354):55–62.
Kong WJ, Wang JB, Xiao XH, Chen SL, Yang MH. Evaluation of antibacterial effect and mode of Coptidis rhizoma by microcalorimetry coupled with chemometric techniques. Analyst. 2012;137(1):216–22. doi:10.1039/c1an15826k.
Kong WJ, Zhao YL, Xing XY, Ma XP, Sun XJ, Yang MH, et al. Antibacterial evaluation of flavonoid compounds against E-coli by microcalorimetry and chemometrics. Appl Microbiol Biotechnol. 2015;99(14):6049–58. doi:10.1007/s00253-015-6711-1.
Semenitz E. Microcalorimetric investigations, regarding the antibacterial efficiency of chemotherapeutics (author’s transl). Immun Infekt. 1978;6(6):260–6.
Kong WJ, Xing XY, Xiao XH, Zhao YL, Wei JH, Wang JB, et al. Effect of berberine on Escherichia coli, Bacillus subtilis, and their mixtures as determined by isothermal microcalorimetry. Appl Microbiol Biotechnol. 2012;96(2):503–10. doi:10.1007/s00253-012-4302-y.
Zhao YL, Liu SX, Qu F, Wang JB, Hu Y, Zhang P, et al. Microcalorimetry coupled with principal component analysis for investigating the anti-Staphylococcus aureus effects of different extracted fractions from Dracontomelon dao. J Therm Anal Calorim. 2015;120(1):913–20. doi:10.1007/s10973-014-4268-3.
Nava DE, Gomez-Torres ML, Rodrigues MD, Bento JM, Haddad ML, Parra JR. The effects of host, geographic origin, and gender on the thermal requirements of Diaphorina citri (Hemiptera: Psyllidae). Environ Entomol. 2010;39(2):678–84. doi:10.1603/EN09252.
Van Vuuren DP, Meinshausen M, Plattner GK, Joos F, Strassmann KM, Smith SJ, et al. Temperature increase of 21st century mitigation scenarios. Proc Natl Acad Sci USA. 2008;105(40):15258–62. doi:10.1073/pnas.0711129105.
McMahon R, Stauffacher M, Knutti R. The unseen uncertainties in climate change: reviewing comprehension of an IPCC scenario graph. Clim Change. 2015;133(2):141–54. doi:10.1007/s10584-015-1473-4.
Johnson LR, Ben-Horin T, Lafferty KD, McNally A, Mordecai E, Paaijmans KP, et al. Understanding uncertainty in temperature effects on vector-borne disease: a Bayesian approach. Ecology. 2015;96(1):203–13.
Barton BT. Local adaptation to temperature conserves top-down control in a grassland food web. Proc Biol Sci R Soc. 2011;278(1721):3102–7. doi:10.1098/rspb.2011.0030.
Walton WE, Workman PD, Tempelis CH. Dispersal, survivorship, and host selection of Culex erythrothorax (Diptera: Culicidae) associated with a constructed wetland in southern California. J Med Entomol. 1999;36(1):30–40.
Leppik EE. Phylogeny, hologeny and coenogeny, basic concepts of environmental biology. Acta Biotheor. 1974;23(3–4):170–93.
Downs WG. The known and the unknown in yellow fever ecology and epidemiology. Ecol Dis. 1982;1(2–3):103–10.
Bidlingmayer WL. The measurement of adult mosquito population changes–some considerations. J Am Mosq Control Assoc. 1985;1(3):328–48.
Rea JG, Irwin SW. The ecology of host-finding behaviour and parasite transmission: past and future perspectives. Parasitology. 1994;109(Suppl):S31–9.
Leather SR. Influential entomology: a short review of the scientific, societal, economic and educational services provided by entomology. Ecol Entomol. 2015;40:36–44. doi:10.1111/een.12207.
Ismail M, Vernon P, Hance T, Pierre JS, van Baaren J. What are the possible benefits of small size for energy-constrained ectotherms in cold stress conditions? Oikos. 2012;121(12):2072–80. doi:10.1111/j.1600-0706.2012.20582.x.
Chiyaka C, Singer BH, Halbert SE, Morris JG Jr, van Bruggen AH. Modeling huanglongbing transmission within a citrus tree. Proc Natl Acad Sci USA. 2012;109(30):12213–8. doi:10.1073/pnas.1208326109.
Munyaneza JE, Sengoda VG, Buchman JL, Fisher TW. Effects of temperature on ‘Candidatus Liberibacter solanacearum’ and Zebra Chip Potato Disease Symptom Development. Plant Dis. 2012;96(1):18–23. doi:10.1094/Pdis-03-11-0185.
Kong W, Zhao Y, Shan L, Xiao X, Guo W. Microcalorimetric studies of the action on four organic acids in Radix isatidis on the growth of microorganisms. Sheng wu gong cheng xue bao = Chin J Biotechnol. 2008;24(4):646–50.
Johnson MD, Volker J, Moeller HV, Laws E, Breslauer KJ, Falkowski PG. Universal constant for heat production in protists. Proc Natl Acad Sci USA. 2009;106(16):6696–9. doi:10.1073/pnas.0902005106.
Neven LG. Physiological effects of physical postharvest treatments on insects. Horttechnology. 2003;13(2):272–5.
McMillan DM, Fearnley SL, Rank NE, Dahlhoff EP. Natural temperature variation affects larval survival, development and Hsp70 expression in a leaf beetle. Funct Ecol. 2005;19(5):844–52. doi:10.1111/j.1365-2435.2005.01031.x.
Dahlhoff EP, Fearnley SL, Bruce DA, Gibbs AG, Stoneking R, McMillan DM, et al. Effects of temperature on physiology and reproductive success of a montane leaf beetle: implications for persistence of native populations enduring climate change. Physiol Biochem Zool. 2008;81(6):718–32. doi:10.1086/590165.
Shi BK, Huang JL, Hu CX, Hou ML. Interactive effects of elevated CO2 and temperature on rice planthopper, Nilaparvata lugens. J Integr Agric. 2014;13(7):1520–9. doi:10.1016/S2095-3119(14)60804-2.
Dallas HF, Ross-Gillespie V. Sublethal effects of temperature on freshwater organisms, with special reference to aquatic insects. Water SA. 2015;41(5):712–26. doi:10.4314/wsa.v41i5.15.
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL. Effects of size and temperature on metabolic rate. Science. 2001;293(5538):2248–51. doi:10.1126/science.1061967.
Clarke A, Fraser KPP. Why does metabolism scale with temperature? Funct Ecol. 2004;18(2):243–51. doi:10.1111/j.0269-8463.2004.00841.x.
Dillon ME, Frazier MR. Thermodynamics constrains allometric scaling of optimal development time in insects. PLoS ONE. 2013;. doi:10.1371/journal.pone.0084308.
Davidson G, Phelps K, Sunderland KD, Pell JK, Ball BV, Shaw KE, et al. Study of temperature-growth interactions of entomopathogenic fungi with potential for control of Varroa destructor (Acari: Mesostigmata) using a nonlinear model of poikilotherm development. J Appl Microbiol. 2003;94(5):816–25.
Regniere J, Powell J, Bentz B, Nealis V. Effects of temperature on development, survival and reproduction of insects: Experimental design, data analysis and modeling. J Insect Physiol. 2012;58(5):634–47. doi:10.1016/j.jinsphys.2012.01.010.
Blanckenhorn WU, Gautier R, Nick M, Puniamoorthy N, Schafer MA. Stage- and sex-specific heat tolerance in the yellow dung fly Scathophaga stercoraria. J Therm Biol. 2014;46:1–9. doi:10.1016/j.jtherbio.2014.09.007.
Blanckenhorn WU, Briegel U, Choffat Y, Demont M, Gautier R, Pemberton KL, et al. Temperature-mediated microhabitat choice and development time based on the pgm locus in the yellow dung fly Scathophaga stercoraria. Biol J Linn Soc. 2012;107(3):686–96. doi:10.1111/j.1095-8312.2012.01955.x.
Scharf I, Bauerfeind SS, Blanckenhorn WU, Schafer MA. Effects of maternal and offspring environmental conditions on growth, development and diapause in latitudinal yellow dung fly populations. Clim Res. 2010;43(1–2):115–25. doi:10.3354/cr00907.
Blanckenhorn WU, Henseler C. Temperature-dependent ovariole and testis maturation in the yellow dung fly. Entomol Exp Appl. 2005;116(3):159–65. doi:10.1111/j.1570-7458.2005.00316.x.
Bale JS, Hayward SAL. Insect overwintering in a changing climate. J Exp Biol. 2010;213(6):980–94. doi:10.1242/jeb.037911.
Berg MP, Kiers ET, Driessen G, van der Heijden M, Kooi BW, Kuenen F, et al. Adapt or disperse: understanding species persistence in a changing world. Glob Change Biol. 2010;16(2):587–98. doi:10.1111/j.1365-2486.2009.02014.x.
Colgan LJ, Erbilgin N. The ecological interaction of the mountain pine beetle and jack pine budworm in the boreal forest. For Chron. 2010;86(6):766–74.
Le Ralec A, Anselme C, Outreman Y, Poirie M, van Baaren J, Le Lann C, et al. Evolutionary ecology of the interactions between aphids and their parasitoids. C R Biol. 2010;333(6–7):554–65. doi:10.1016/j.crvi.2010.03.010.
Prokop J, Wappler T, Knor S, Kvacek Z. Plant-arthropod associations from the lower miocene of the most basin in Northern Bohemia (Czech Republic): a preliminary report. Acta Geol Sin-Engl. 2010;84(4):903–14.
Rojas MR, Locatelli B, Billings R. Climate change and outbreaks of Southern Pine Beetle Dendroctonus frontalis in Honduras. For Syst. 2010;19(1):70–6.
Romo CM, Tylianakis JM. Elevated temperature and drought interact to reduce parasitoid effectiveness in suppressing hosts. PLoS ONE. 2013;. doi:10.1371/journal.pone.0058136.
Post E, Pedersen C. Opposing plant community responses to warming with and without herbivores. Proc Natl Acad Sci USA. 2008;105(34):12353–8. doi:10.1073/pnas.0802421105.
Chung H, Muraoka H, Nakamura M, Han S, Muller O, Son Y. Experimental warming studies on tree species and forest ecosystems: a literature review. J Plant Res. 2013;126(4):447–60. doi:10.1007/s10265-013-0565-3.
Jamieson MA, Schwartzberg EG, Raffa KF, Reich PB, Lindroth RL. Experimental climate warming alters aspen and birch phytochemistry and performance traits for an outbreak insect herbivore. Glob Change Biol. 2014;. doi:10.1111/gcb.12842.
Bauerfeind SS, Fischer K. Increased temperature reduces herbivore host-plant quality. Glob Change Biol. 2013;19(11):3272–82. doi:10.1111/gcb.12297.
Lu X, Siemann E, Shao X, Wei H, Ding J. Climate warming affects biological invasions by shifting interactions of plants and herbivores. Glob Change Biol. 2013;19(8):2339–47. doi:10.1111/gcb.12244.
Robinet C, Roques A. Direct impacts of recent climate warming on insect populations. Integr Zool. 2010;5(2):132–42. doi:10.1111/j.1749-4877.2010.00196.x.
Yang S, Ruuhola T, Haviola S, Rantala MJ. Temperature as a modifier of plant-herbivore interaction. J Chem Ecol. 2007;33(3):463–75. doi:10.1007/s10886-006-9239-0.
Imbahale SS, Paaijmans KP, Mukabana WR, van Lammeren R, Githeko AK, Takken W. A longitudinal study on Anopheles mosquito larval abundance in distinct geographical and environmental settings in western Kenya. Malar J. 2011;10:81. doi:10.1186/1475-2875-10-81.
Wu GM, Barrette M, Boivin G, Brodeur J, Giraldeau LA, Hance T. Temperature influences the handling efficiency of an aphid parasitoid through body size-mediated effects. Environ Entomol. 2011;40(3):737–42. doi:10.1603/EN11018.
Tu Z, Ling B, Xu D, Zhang M, Zhou G. Effects of southern rice black-streaked dwarf virus on the development and fecundity of its vector, Sogatella furcifera. Virol J. 2013;10:145. doi:10.1186/1743-422X-10-145.
Christiansen-Jucht C, Parham PE, Saddler A, Koella JC, Basanez MG. Temperature during larval development and adult maintenance influences the survival of Anopheles gambiae ss. Parasites Vectors. 2014;7:489. doi:10.1186/s13071-014-0489-3.
Hall DG, Wenninger EJ, Hentz MG. Temperature studies with the Asian citrus psyllid, Diaphorina citri: cold hardiness and temperature thresholds for oviposition. J Insect Sci. 2011;11:83. doi:10.1673/031.011.8301.
Lopez-Collado J, Isabel Lopez-Arroyo J, Robles-Garcia PL, Marquez-Santos M. Geographic distribution of habitat, development, and population growth rates of the Asian citrus psyllid, Diaphorina citri, in Mexico. J Insect Sci. 2013;13:114. doi:10.1673/031.013.11401.
Acknowledgements
We are thankful to Bao Lu, Zao Yufen and Ru Xinhui for their kind assistance. The authors gratefully acknowledge the financial support of the National Nature Science Foundation of China (31371998), research grants from the Department of Science and Technology of Fujian Province (2016N0005) and the Research Fund for the Doctoral Program of Higher Education of China (20123515110003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hussain, M., Lin, Y. & Wang, L. Effect of temperature on longevity of Diaphorina citri (Hemiptera: Liviidae) studied by microcalorimeter. J Therm Anal Calorim 127, 1245–1252 (2017). https://doi.org/10.1007/s10973-016-5732-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-016-5732-z