Abstract
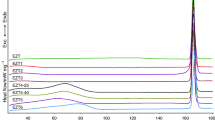
Clozapine is an antipsychotic drug used for refractory schizophrenia and severe psychiatric disorders associated with several side effects. Studies on standardization of raw material and bulk products are necessary to ensure reproducibility batch to batch during all stages of the industrial pharmaceutical process. The aim of this study was to conduct studies of polymorphic characterization and compatibility study of clozapine. Different solvatomorphic forms of clozapine were obtained by recrystallization technique. Polymorphic characterization was performed using optical microscopy, SEM, intrinsic dissolution, and thermal analysis. Compatibility studies of clozapine:excipients were performed by TG and DSC techniques. The polymorphic characterization obtained by analytical and thermal techniques showed the formation of a solvatomorphic form of clozapine (clozapine monohydrate) when recrystallized in aqueous solvents and in alkaline medium. The polymorphic form (clozapine anhydrate) showed higher intrinsic dissolution rate compared to solvatomorphic form (clozapine monohydrate). All industrial batches of clozapine presented in anhydrate form. The DSC/TG data demonstrated similar melting peaks for 2 polymorphic forms, but desolvation peaks characteristic of monohydrate form was observed in clozapine monohydrate. Studies of binary mixtures showed no incompatibilities between clozapine and excipients, except for clozapine:lactose which can reduce the stability of bulk and tablets of clozapine. Tablets of clozapine presented the same thermal analysis profile of clozapine:lactose but did not contribute in decreasing shelf life of clozapine tablets before 24 months of storage. Dissolution studies of the tablets did not show variability between batches of clozapine during 24 months but presented decreasing on stability for 36 months of storage.







Similar content being viewed by others
References
BRASIL, Farmacopéia Brasileira 5ª Edição Vol. 2. Brasília: Df; Dou Nº 224, 24 de novembro de 2010.
Manu P, Sarpal D, Muir O, Kane JM, Corell CU. When can patients with potentially life-threatening adverse effects be rechallenged with clozapine? A systematic review of the published literature. Schizophr Res. 2012;134:180–6.
Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58:265–78.
Stahl SM. Antipsychotics and mood stabilizer, third ed. New York: Cambridge University Press, chapter. 45, 2008. In: Rang HP and Dale MM. Farmacologia. 2011; Rio de Janeiro: Elsevier, 768p.
Ferrari M, Bolla E, Bortolaso P, Callegari C, Poloni N, Lecchini S, Vender S, Marino F, Cosentino M. Association between CYP1A2 polymorphisms and clozapine-induced adverse reactions in patients with schizophrenia. Psychiatry Res. 2012;200:1014–7.
Nascimento TG, Basílio ID Jr, Macedo RO, Moura E, Dornelas C, Bernardo V, Rocha V, Nóvak C. Characterization of the Indinavir raw in some pharmaceutical processes. J Therm Anal Calorim. 2010;102:269–75.
Da Silva RMF, Medeiros FPM, Nascimento TG, Macêdo RO, Neto PJR. Thermal characterization of indinavir sulfate using TG, DSC and DSC-photovisual. J Therm Anal Calorim. 2009;95(3):965–8.
Sehi S, Betz G, Hadzidedi S, El-Arini SK, Leuenberger H. Investigation of intrinsic dissolution behavior of different carbamazepine samples. Int J Pharm. 2010;386:77–90.
Censi R, Martena V, Hoti E, Malaj L, Di Martino P. Sodium ibuprofen dihydrate and anhydrous. J Therm Anal Calorim. 2013;111(3):2009–18.
Chienga N. An overview of recent studies on the analysis of pharmaceutical polymorphs. J Pharm Biomed Anal. 2011;55:618–44.
Böer TM, Procópio JVV, Nascimento TG, Macêdo RO. Correlation of thermal analysis and pyrolysis coupled to GC MS in the characterization of tacrolimus. J Pharm Biomed Anal (Print). 2013;73:18–23.
Agnihotri SA, Aminabhavi TM. Controlled release of clozapine through chitosan microparticles prepared by a novel method. J Control Release. 2004;96:245–59.
Zeng F, Wang L, Zhang W, Shi K, Zong L. Formulation and in vivo evaluation of orally disintegrating tablets of clozapine/hydroxypropyl-β-cyclodextrin inclusion complexes. AAPS PharmSciTech. 2013;14(2):854–60.
Lima NGPB, Lima IPB, Barros DMC, Oliveira TS, Raffin FN, Moura TFAL, Medeiros ACD, Gomes APB, Aragão CFS. Compatibility studies of trioxsalen with excipients by DSC, DTA, and FTIR. J Therm Anal Calorim. 2013; doi:10.1007/s10973-013-3216-y.
Lavor EP, Navarro MV, Freire FD, Aragão CFS, Raffin FN, Barbosa EG, Moura TFA. Application of thermal analysis to the study of antituberculosis drugs–excipient compatibility. J Therm Anal Calorim. 2012;108:207–12.
Santos AF, Basílio ID Jr, de Souza FS, Medeiros AFD, MF Pinto, de Santana DP, Macedo RO. Application of thermal analysis of binary mixtures with metformin. J Therm Anal Calorim. 2008;93(2):361–4.
Soares MFLR, Soares-Sobrinho JL, Silva KER, Alves LDS, Lopes PQ, Correia LP, Souza FS, Macêdo RO, Neto PJR. Thermal characterization of antimicrobial drug ornidazole and its compatibility in a solid pharmaceutical product. J Therm Anal Calorim. 2011;104:307–13.
Medeiros AFD, Santos AFO, Souza FS, Procópio JVV, Pinto MF, Macedo RO. Thermal Stability of paracetamol and its pre-formulates obtained by spray drying. J Therm Anal Calorim. 2007;88(2):377–82.
Joiris E, Di Martino P, Malaj L, Censi R, Barthelemy C, Odou P. Influence of crystal hydration on the mechanical properties of sodium naproxen. Eur J Pharm Biopharm. 2008;70:345–56.
Sun C, Grant DJW. Improved tableting properties of p-hydroxybenzoic acid by water of crystallization: a molecular insight. Pharm Res. 2004;21:382–6.
Malaj L, Cens R, Gashi Z, Di Martino P. Compression behaviour of anhydrous and hydrate forms of sodium naproxen. Int J Pharm. 2010;390:142–9.
Vogt M, Kunath K, Dressman JB. Dissolution improvement of four poorly water soluble drugs by cogrinding with commonly used excipients. Eur J Pharm Biopharm. 2008;68:330–7.
Mosharraf M, Nyström C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int J Pharm. 1995;122:35–47.
Yu LX, Carlin AS, Amidon GL, Hussain AS. Feasibility studies of utilizing disk intrinsic dissolution rate to classify drugs. Int J Pharm. 2004;270:221–7.
Vrecer F, Vrbinc M, Meden A. Characterization of piroxicam crystal modifications. Int J Pharm. 2003;256:3–15.
Kobayashi Y, Ito S, Itai S, Yanamoto K. Physicochemical properties and bioavailability of carbamazepine polymorphs and hydrate. Int J Pharm. 2000;193:137–46.
Zakeri-Milani P, Barzegar-Jalali M, Azimi M, Valizadeh H. Biopharmaceutical classification of drugs using intrinsic dissolution rate (IDR)and rat intestinal permeability. Eur J Pharm Biopharm. 2009;73:102–6.
Buckley ST, Frank KJ, Fricker G, Brandl M. Biopharmaceutical classification of poorly soluble drugs with respect to ‘‘enabling formulations’’. Eur J Pharm Sci. 2013;50:8–16.
Willmann S, Thelen K, Becker C, Dressman JB, Lippert J. Mechanism-based prediction of particle size-dependent dissolution and absorption: cilostazol pharmacokinetics in dogs. Eur J Pharm Biopharm. 2012;76:83–94.
Berlin E, Kliman PG, Anderson BA, Pallansch MJ. Calorimetric measurement of the heat of desorption of water vapor from amorphous and crystalline lactose. Thermochim Acta. 1971;2:143–52.
Macêdo RO, Nascimento TG, Veras JWE. Comparison of generic hydrochlorothiazide formulations by means of TG and DSC coupled to a photovisual system. J Therm Anal Calorim. 2001;64:757–63.
Acknowledgements
The authors thank to the CAPES and CNPq for its financial support with Grant Number 478390/2010-06 of the funding of research no 14/2010—Universal/MS/CNPq. The authors thank to FINEP for its financial support (CT-INFRA) in acquisition of some equipments and laboratorial facilities. The authors also want to thank to the Pharmaceutical Industrial Laboratory of Alagoas (LIFAL) in the persons of Vânia do Nascimento Rocha, manager of the department of quality assurance, Solange S. Moura and Denise M. de Melo França pharmacists and Maria Cícera C. Santos for the technical support in the selection of the raw materials and Intrinsic Dissolution and dissolution profile and Ana Rúbia Ribeiro for the support in the SEM analysis at the Institute of Physics from Federal University of Alagoas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dias, S.B.T., Nascimento, T.G., Santos, A.F.O. et al. Polymorphic characterization and compatibility study of clozapine: implications on its stability and some biopharmaceutics properties. J Therm Anal Calorim 120, 795–805 (2015). https://doi.org/10.1007/s10973-014-4142-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-014-4142-3