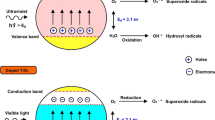
Abstract
The influence of synthesis parameters using the ultrasonic-assisted sol–gel method on the properties of TiO2 nanostructures was studied. Different synthesis conditions such as long stirring time, type of acid used as the catalyst (weak or strong acid), and calcination temperature were explored and their influences on the morphology, size, crystallinity of nanostructures, and photocatalytic performance were evaluated. TiO2 nanostructures were characterized by Fourier transform infrared spectroscopy, transmission electron microscopy, X-ray diffraction, and ultraviolet–visible diffuse reflectance spectroscopy. Moreover, degradation photocatalytic tests were performed using methylene blue as the polluting model. Changes in the morphologies and sizes of nanostructures were observed by modification of the stirring time using this sonication process. Moreover, the use of a weak acid, such as acetic acid, produced nanostructures with higher anatase proportion, larger crystallite size, and larger nanoparticle size compared with the use of a strong acid such as nitric acid. In addition, changes in crystalline phases were observed with an increase in the calcination temperature. Finally, the photocatalytic performance was influenced mainly by the changes in the crystalline phases, which were dependent on the type of acid catalyst and the calcination temperature.

Highlights
-
Synthesis parameters of TiO2 nanostructures by the sol–gel method are investigated.
-
Ultrasonic stirring time, acid type as a catalyst and calcination temperature are evaluated.
-
Morphology, size, crystallinity, and photocatalytic performance of nanostructures are studied.
-
Ultrasonic stirring time affect the morphology and the size of nanostructure.
-
Acid type and calcination temperature influence on crystalline phases and nanoparticle size.
-
Photocatalytic performance is mainly influenced by the change in the crystalline phases.





Similar content being viewed by others
References
Mahshid S, Askari M, Ghamsari M (2007) Synthesis of TiO2 nanoparticles by hydrolysis and peptization of titanium isopropoxide solution. J Mater Process Technol 189:296–300
Lopez-Muñoz M, Revilla A, Alcalde G (2015) Brookite TiO2-based materials: synthesis and photocatalytic performance in oxidation of methyl orange and As(III) in aqueous suspensions. Catal Today 240:138–145
Afuyoni M, Nashed G, Nasser I (2011) TiO2 doped with SnO2 and studing its structurail and electrical properties. Energy Procedia 6:11–20
García A, Quintero Y, Vicencio N, Rodríguez B, Ozturk D, Mosquera E, Corrales TP, Volkmann UG (2016) Influence of TiO2 nanostructures on anti-adhesion and photoinduced bactericidal properties of thin film composite membranes. RSC Adv 6:82941–82948
Liu J, Li Y, Arumugam S, Tudor J, Beeby S (2018) Investigation of low temperature processed titanium dioxide (TiO2) films for printed dye sensitized solar cells (DSSCs) for large area flexible applications. Mater Today 5:13846–13854
Omprakash S, Kumar S, Holla R (2018) Titanium dioxide and Zinc oxide as a dielectric material for application in TFT’s. Mater Today 5:10833–10838
H. Memon, S. Yasin, N. Khoso, M. Hussain (2015) Indoor decontaminating textiles by photo catalytic oxidation—a review. J Nanotech 2015:1–9
Memon H, Kumari N (2016) Study of multifunctional nanocoated cold plasma treated polyester cotton blended curtains. Surf Rev Lett 5:1–11
Chen W, Yu J, Hu W, Chen Z, Memon H, Chen G (2016) Titanate nanowires/NiO nanoflakes core/shell heterostructured nanonanocomposite catalyst for the methylene blue photodegradation. RSC Adv 72:67827–67832
Saini A, Sharma J, Sharma R, Chaudhary A, Sharma D, Dhayal V (2019) Zinc oxide derived from zinc(II)/acetoxime system: formation pathway and solar-driven photocatalytic and antimicrobial applications. J Sol-Gel Sci Technol 91:644–653
Memon H, Kumari N, Jatoi A, Khoso N (2016) Study of the indoor decontamination using nanocoated woven polyester fabric. Int Nano Lett 7:1–7
Baradaran M, Ghodsi F (2019) Highly efficient visible photocatalytic degradation of MB organic dye by heteromorphic ZnO/AZO/ZnO nanocatalysts: effect of AZO thickness. J Sol–Gel Sci Technol 92:25–39
Dubey R (2018) Temperature-dependent phase transformation of TiO2 nanoparticles synthesized by sol-gel method. Mater Lett 215:312–317
Imran M, Riaz S, Naseem S (2015) Synthesis and characterization of titania nanoparticles by sol-gel technique. Mater Today 2:5455–5461
Kanna M, Wongnawa S (2008) Mixed amorphous and nanocrystalline TiO2 powders prepared by sol–gel method: characterization and photocatalytic study. Mater Chem Phys 110:166–175
Jongprateep O, Puranasamriddhi R (2018) Effects of reagents on the formation of nanoparticulatetitaniumdioxide synthesized by sol-gel technique. Mater Today 5:10925–10931
Ma Z, Jiang Y, Xiao H, Jiang B, Zhang H, Peng M, Dong G, Yu X, Yang J (2018) Sol-gel preparation of Ag-silica nanocomposite with high electrical conductivity. Appl Surf Sci 436:732–738
Kong F, Ning W, Wang A, Liu Y, Tian M (2018) Convenient solvothermal synthesis of nanoscale 0-2D Bi without surfactants and templates. J Alloy Compd 737:484–489
Bincy J, Silvena G, Rajesh A (2018) Influence of reaction time on the structural, optical and electrical performance of copper antimony sulfide nanoparticles using solvothermal method. Phys B 537:243–250
Meng L, Wang B, Ma M, Lin K (2016) The progress of microwave-assisted hydrothermal method in the synthesis of functional nanomaterials. Mater Today Chem 1:63–83
Peng T, Ray S, Veeravalli S, Lalman J, Arefi F (2018) The role of hydrothermal conditions in determining 1D TiO2 nanomaterials bandgap energies and crystal phases. Mater Res Bull 105:104–113
Goyal A, Soni P (2018) Functionally graded nanocrystalline silicon powders by mechanical alloying. Mater Lett 214:111–114
Rabiee M, Mirzadeh H, Ataie A (2017) Processing of Cu-Fe and Cu-Fe-SiC nanocomposites by mechanical alloying. Adv Powder Technol 28:1882–1887
Tan D, Zhou S, Qiu J, Khusro N (2013) Preparation of functional nanomaterials with femtosecond laser ablation in solution. J Photochemistry Photobiol C 17:50–68
Xiao J, Liu P, Wang C, Yang G (2017) External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog Mater Sci 87:140–220
Pelaez M, Nolan N, Pillai S, Seery M, Falaras P, Kontos A, Dunlop P, Hamilton J, Byrne J, O’Shea K, Entezari M, Dionysiou D (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B 125:331–349
Mahy J, Deschamps F, Collard V, Jérôme C, Bartlett J, Lambert S, Heinrichs B (2018) Acid acting as redispersing agent to form stable colloids from photoactive crystalline aqueous sol–gel TiO2 powder. J Sol–Gel Sci Technol 87:568–583
Tian Q, Wei W, Dai J, Sun Q, Zhuang J, Zheng Y, Liu P, Fan M, Chen L (2019) Porous core-shell TixSn1-xO2 solid solutions with broad-light response: one-pot synthesis and ultrahigh photooxidation performance. Appl Catal B 244:45–55
Sayilkan F, Asiltürk M, Sayilkan H, Önal Y, Akarsu M, Apac E (2005) Characterization of TiO2 synthesized in alcohol by a sol-gel process: the effects of annealing temperature and acid catalyst. Turk J Chem 29:697–706
Andrade M, Díaz L, Cortés D, Cabello C, Ávila C, Bartolo P, Gamero P (2018) Microwave assisted sol–gel synthesis of titanium dioxide using hydrochloric and acetic acid as catalyst. Boletín de la sociedad Española de céramica y vidrio. https://doi.org/10.1016/j.bsecv.2018.10.005
Arunmetha S, Manivasakan P, Karthik A, Babu N, Srither S, Rajendran V (2013) Effect of processing methods on physicochemical properties of titania nanoparticles produced from natural rutile sand. Adv Powder Technol 24:972–979
Xia X, Luo Y, Wang Z, Liang Y, Fan J, Jia Z, Chen Z (2007) Ultrasonic synthesis and photocatalytic activity investigation of TiO2 nanoarrays. Mater Lett 61:2571–2574
Neppolian B, Wang Q, Jung H, Choi H (2008) Ultrasonic-assisted sol-gel method of preparation of TiO2 nano-particles: Characterization, properties and 4-chlorophenol removal application. Ultrason Sonochem 15:649–658
Li B, Wang X, Yan M, Li L (2002) Preparation and characterization of nano-TiO2 powder. Mater Chem Phys 78:184–188
Jenkins R, Snyder R (1996) Introduction to X-ray powder diffractometry. vol 138. Wiley, New York, NY
Kubelka P, Munk F (1931) An article on optics of paint layers. Tech Phys 12:593
Kenneth P, Suslick S, Gareth J (1999) Applicatios of ultrasound to materials chemistry. Annu Rev Mater Sci 29:295–326
ullah H, Khan I, Yamani Z, Qurashi A (2017) Sonochemical-driven ultrafast facile synthesis of SnO2 nanoparticles: Growth mechanism structural electrical and hydrogen gas sensing properties. Ultrason Sonochem 34:484–490
Mosquera E, Carvajal N, Morel M, Marín C (2017) Fabrication of ZnSe nanoparticles: structural, optical and Raman Studies. J Lumin 192:814–817
Mosquera E, Carvajal N (2014) Low temperature synthesis and blue photoluminescence of ZnS submicronparticles. Mater Lett 129:8–11
Alzamani M, Shokuhfar A, Eghdam E, Mastali S (2013) Influence of catalyst on structural and morphological properties of TiO2 nanostructured films prepared by sol–gel on glass. Prog Nat Sci 1:77–84
Naseem S, Riaz S (2015) Controlled nanostructuring of TiO2 nanoparticles: a sol-gel approach. J Sol–Gel Sci Technol 74:299–309
Mathew S, Kumar A, Benoy T, Rakesh P, Hari M, Libish T, Radhakrishnan P, Nampoori V, Vallabhan C (2012) UV-visible photoluminiscence of TiO2 nanoparticles prepared by hydrothermal method. J Fluoresc 22:1563–1569
Vinogradov A, Vinogradov V (2014) Effect of acidic peptization on formation of highly photoactive TiO 2 films prepared without heat treatment. J Am Ceram Soc 97:290–294
Malengreaux C, Pirard S, Léonard G, Mahy J, Herlitschke M, Klobes B, Hermann R, Heinrichs B, Bartlett J (2017) Study of the photocatalytic activity of Fe3+, Cr3+, La3+ and Eu3+ single-doped and co-doped TiO2 catalysts produced by aqueous sol-gel processing. J Alloy Compd 691:726–738
Reyes-Coronado D, Rodriguez-Gattorno G, Espinosa-Pesqueira ME, Cab C, de Coss R, Oskam G (2008) phase-pure TiO2 nanoparticles: anatase, brookite and rutile. Nanotechnology 19:145605
Matos J, Quintana K, García A (2012) Influence of H-type and L-type activated carbon in the photodegradation of methylene blue and phenol under UV and visible light irradiated TiO2. Mod Res Catal 1:1–9
Acknowledgements
The authors gratefully acknowledge the financial support provided by the Fund for the Promotion of Scientific and Technological Development (FONDEF) of the Government of Chile (Project no. ID15I10086) and also acknowledge the Universidad de Santiago de Chile (USACH), especially Professor Diego Venegas-Yazigi for the UV–vis/DRS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Quintero, Y., Mosquera, E., Diosa, J. et al. Ultrasonic-assisted sol–gel synthesis of TiO2 nanostructures: Influence of synthesis parameters on morphology, crystallinity, and photocatalytic performance. J Sol-Gel Sci Technol 94, 477–485 (2020). https://doi.org/10.1007/s10971-020-05263-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05263-6