Abstract
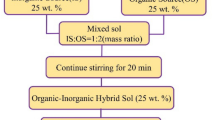
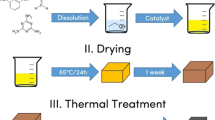
Resorcinol–formaldehyde (RF) aerogels are widely used as thermal insulation materials. The present investigation of RF aerogels emphases on improving hydrophobic property and high-temperature residual yields. In this study, polysiloxane-modified RF composite xerogel monoliths were prepared by gelation of poly(methylphenylsiloxane) prepolymer with RF in cosolvent and ambient pressure drying. The influence of phenyl content on structure and thermal property of xerogels was investigated. The as-prepared RF composite monoliths were lightweight and hydrophobic with density lower than 0.300 g/cm3 and water contact angles approaching 130°. Morphology analyses indicated that polysiloxane grew and formed a continuous layer on RF porous skeleton with controlled reaction parameter. Thermogravimetry analyses (TGA) certified that the controlled incorporation of polysiloxane in composite xerogels enhanced the thermal degradation temperature at which the weight loss of RF xerogels reaches 5% (T5%) and increased the residual yields at 1000 °C. The carbonized derivatives were obtained for further enhancing the thermal stability by removal of small molecules during carbonization. Xerogel monoliths preserved hydrophobic property till 500 °C carbonization due to the thermal stability of poly(methylphenylsiloxane). The heat-resistant composite xerogels remained monolith structure after 800 °C treatment under nitrogen condition.

The prepared composite xerogels were lightweight, hydrophobic, and thermal resistant. The hydrophobic property was preserved for 500 °C-carbonized derivatives. The heat-resistant composite xerogels remained monolith structure after 800 °C carbonized treatment under nitrogen condition.
Highlights
-
Heat-resistant and hydrophobic xerogel monoliths were prepared by ambient drying.
-
The temperature at which the weight loss of RF xerogels reaches 5% was increased by the controlled incorporation of poly(methylphenylsiloxane).
-
Composite xerogels preserved hydrophobicity after 500 °C carbonization.
-
Macroscale separation of polysiloxane and resorcinol–formaldehyde was resisted.











Similar content being viewed by others
References
Park JK, Kang TJ (2002) Thermal and ablative properties of low temperature carbon fiber-phenol formaldehyde resin composites. Carbon 40(12):2125–2134
Pekala RW (1989) Organic aerogels from the polycondensation of resorcinol with formaldehyde. J Mater Sci 24(9):3221–3227
Lin C, Ritter JA (1997) Effect of synthesis pH on the structure of carbon xerogels. Carbon 35(9):1271–1278
Tamon H, Ishizaka H (2000) Influence of gelation temperature and catalysts on the mesoporous structure of resorcinol-formaldehyde aerogels. J Colloid Interface Sci 223(2):305–307
Job N, Théry A, Pirard R, Marien J, Kocon L, Rouzaud JN, Béguin F, Pirard JP (2005) Carbon aerogels, cryogels and xerogels: Influence of the drying method on the textural properties of porous carbon materials. Carbon 43(12):2481–2494
Qin G, Wei W, Guo S (2003) Semi-continuous drying of RF gels with supercritical acetone. Carbon 41(4):851–853
Alonso-Buenaposada ID, Rey-Raap N, Calvo EG, Menéndez JA, Arenillas A (2017) Acid-based resorcinol-formaldehyde xerogels synthesized by microwave heating. J Sol–Gel Sci Technol 84(1):60–69
Alshrah M, Mark LH, Zhao C, Naguib HE, Park CB (2018) Nanostructure to thermal property relationship of resorcinol formaldehyde aerogels using the fractal technique. Nanoscale 10(22):10564–10575
Rey-Raap N, Calvo EG, Menéndez JA, Arenillas A (2017) Exploring the potential of resorcinol-formaldehyde xerogels as thermal insulators. Microporous Mesoporous Mater 244:50–54
Wiener M, Reichenauer G, Braxmeier S, Hemberger F, Ebert HP (2009) Carbon aerogel-based high-temperature thermal insulation. Int J Thermophys 30(4):1372–1385
Cui S, Suo H, Jing F, Yu S, Xue J, Shen X, Lin B, Jiang S, Liu Y (2018) Facile preparation of ZrCO composite aerogel with high specific surface area and low thermal conductivity. J Sol-Gel Sci Technol 86(2):383–390
Laskowski J, Milow B, Ratke L (2016) Aerogel-aerogel composites for normal temperature range thermal insulations. J Non-Cryst Solids 441:42–48
Xu H, Zhang H, Huang Y, Wang Y (2010) Porous carbon/silica composite monoliths derived from resorcinol-formaldehyde/TEOS. J Non-Cryst Solids 356(20–22):971–976
Chen K, Bao Z, Du A, Zhu X, Shen J, Wu G, Zhang Z, Zhou B (2012) One-pot synthesis, characterization and properties of acid-catalyzed resorcinol/formaldehyde cross-linked silica aerogels and their conversion to hierarchical porous carbon monoliths. J Sol–Gel Sci Technol 62(3):294–303
Yu ZL, Yang N, Apostolopoulou-Kalkavoura V, Qin B, Ma ZY, Xing WY, Qiao C, Bergström L, Antonietti M, Yu SH (2018) Fire-retardant and thermally insulating phenolic-silica aerogels. Angew Chem Int Ed 57(17):4538–4542
Liu B, Ju W, Zhang J, Fan H, Wang Q, Yi X, Yu Z, Wang X (2017) Improvement of mechanical strength of ultralight resorcinol-formaldehyde/silica aerogel by addition of zirconia. J Sol–Gel Sci Technol 83(1):100–108
Hüsing N, Schubert U (1998) Aerogels-airy materials: chemistry, structure, and properties. Angew Chem Int Ed 37(1–2):23–45
Alonso-Buenaposada ID, Calvo EG, Montes-Morán MA, Narciso J, Menéndez JA, Arenillas A (2016) Desiccant capability of organic xerogels: surface chemistry vs porous texture. Microporous Mesoporous Mater 232:70–76
Jerman M, Černý R (2012) Effect of moisture content on heat and moisture transport and storage properties of thermal insulation materials. Energy Build 53:39–46
Alonso-Buenaposada ID, Arenillas A, Montes-Morán MA, Menéndez JA (2017) Superhydrophobic and breathable resorcinol-formaldehyde xerogels. J Non-Cryst Solids 471:202–208
Rao AV, Kalesh RR, Amalnerkar DP, Seth T (2003) Synthesis and characterization of hydrophobic TMES/TEOS based silica aerogels. J Porous Mater 10(1):23–29
Alonso-Buenaposada ID, Montes-Morán MA, Angel Menéndez J, Arenillas A (2017) Synthesis of hydrophobic resorcinol-formaldehyde xerogels by grafting with silanes. React Funct Polym 120:92–97
Wu Q, Zhang Q, Zhao L, Li SN, Wu LB, Jiang JX, Tang LC (2017) A novel and facile strategy for highly flame retardant polymer foam composite materials: Transforming silicone resin coating into silica self-extinguishing layer. J Hazard Mater 336:222–231
Hamdani S, Longuet C, Perrin D, Lopez-Cuesta JM, Ganachaud F (2009) Flame retardancy of silicone-based materials. Polym Degrad Stab 94(4):465–495
Li S, Han Y, Chen F, Luo Z, Li H, Zhao T (2016) The effect of structure on thermal stability and anti-oxidation mechanism of silicone modified phenolic resin. Polym Degrad Stab 124:68–76
Li S, Li H, Li Z, Zhou H, Guo Y, Chen F, Zhao T (2017) Polysiloxane modified phenolic resin with co-continuous structure. Polymer 120:217–222
Yun J, Chen L, Zhang X, Zhao H, Wen Z, Zhu D (2018) Synthesis and structure evolution of phenolic resin/silicone hybrid composites with improved thermal stability. J Mater Sci 53:14185–14203
Kong Y, Zhong Y, Shen X, Cui S, Yang M, Teng K, Zhang J (2012) Facile synthesis of resorcinol-formaldehyde/silica composite aerogels and their transformation to monolithic carbon/silica and carbon/silicon carbide composite aerogels. J Non-Cryst Solids 358(23):3150–3155
Berthon-Fabry S, Hildenbrand C, Ilbizian P (2016) Lightweight superinsulating resorcinol-formaldehyde-APTES benzoxazine aerogel blankets for space applications. Eur Polym J 78:25–37
Cheng H, Xue H, Hong C, Zhang X (2016) Characterization, thermal and mechanical properties and hydrophobicity of resorcinol-furfural/silicone hybrid aerogels synthesized by ambient-pressure drying. RSC Adv 6(79):75793–75804
Seraji MM, Sameri G, Davarpanah J, Bahramian AR (2017) The effect of high temperature sol-gel polymerization parameters on the microstructure and properties of hydrophobic phenol-formaldehyde/silica hybrid aerogels. J Colloid Interface Sci 493:103–110
Sun JT, Huang YD, Cao HL, Gong GF (2004) Effects of ambient-temperature curing agents on the thermal stability of poly(methylphenylsiloxane). Polym Degrad Stab 85(1):725–731
Seraji MM, Ghafoorian NS, Bahramian AR, Alahbakhsh A (2015) Preparation and characterization of C/SiO2/SiC aerogels based on novolac/silica hybrid hyperporous materials. J Non-Cryst Solids 425:146–152
Worsley MA, Kuntz JD, Satcher JH, Baumann TF (2010) Synthesis and characterization of monolithic, high surface area SiO2/C and SiC/C composites. J Mater Chem 20(23):4840–4844
Zhou NL (2000) Introduction to silicone polymers. Science Press, Beijing, p 150
Prado LASA, Sforça ML, de Oliveira AG, Yoshida IVP (2008) Poly(dimethylsiloxane) networks modified with poly(phenylsilsesquioxane)s: Synthesis, structural characterisation and evaluation of the thermal stability and gas permeability. Eur Polym J 44(10):3080–3086
Gao DH, Jia MQ, Luo Y (2013) Crosslinked organosiloxane hybrid materials prepared by condensation of silanol and modified silica: synthesis and characterization. Chin J Polym Sci 31(7):974–983
Yang Z, Feng L, Diao S, Feng S, Zhang C (2011) Study on the synthesis and thermal degradation of silicone resin containing silphenylene units. Thermochim Acta 521(1–2):170–175
Ou DL, Seddon AB (1997) Near- and mid-infrared spectroscopy of sol-gel derived ormosils: vinyl and phenyl silicates. J Non-Cryst Solids 210(2–3):187–203
Laskowski J, Milow B, Ratke L (2014) Subcritically dried resorcinol-formaldehyde aerogels from a base-acid catalyzed synthesis route. Microporous Mesoporous Mater 197:308–315
Aghabararpour M, Naderi M, Motahari S, Najafi M (2019) A study on resorcinol formaldehyde carbon aerogel/epoxy nanocomposites: the effect of carbon aerogel pyrolysis time. J Polym Res 26(3):59
Chen S, Zhuo D, Hu JT (2018) Sol-gel technology plus radiation curing: a novel and facile technique for preparing thick, large-area hyperbranched polysiloxane hybrids. Ind Eng Chem Res 57(31):10372–10378
Costa L, di Montelera LR, Camino G, Weil ED, Pearce EM (1997) Structure-charring relationship in phenol-formaldehyde type resins. Polym Degrad Stab 56(1):23–35
Gao D, Jia M (2013) Synthesis of poly(methylphenylsiloxane)/phenylene-silica hybrid material with interpenetrating networks and its performance as thermal resistant coating. J Appl Polym Sci 128(6):3619–3630
Yang Z, Han S, Zhang R, Feng S, Zhang C, Zhang S (2011) Effects of silphenylene units on the thermal stability of silicone resins. Polym Degrad Stab 96(12):2145–2151
Camino G, Lomakin SM, Lazzari M (2001) Polydimethylsiloxane thermal degradation Part 1 Kinetic aspects. Polymer 42(6):2395–2402
Yan X, Tsotsis TT, Sahimi M (2015) Fabrication of high-surface area nanoporous SiOC materials using pre-ceramic polymer blends and a sacrificial template. Microporous Mesoporous Mater 210:77–85
Yun S, Guo T, Zhu X, Zhang L, Zhang J, Gao X, Gao Y (2018) Effects of carbonization temperature on structure and mechanical properties of monolithic C/SiO2 aerogels based on ambient pressure dried superhydrophobic resorcinol-formaldehyde/SiO2 aerogels. J Porous Mater 25(6):1825–1830
Tao Y, Li P (2019) Effects of fabrication parameters on the oxidation resistance of wood/phenolic resin carbon composites. Mater tehnologije 53(3):417–423
Wang C, Jin X, Cheng H, Hong C, Zhang X (2017) Organic aerogel-impregnated low-density carbon/carbon composites: preparation, properties and response under simulated atmospheric re-entry conditions. Mater Des 131:177–185
Acknowledgements
The authors gratefully acknowledge the financial support from Heilongjiang Postdoctoral Science-Research Foundation (Grant No.: LBH-Q11099).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, R., Xu, H., Zhong, Z. et al. Hydrophobic and heat-resistant poly(methylphenylsiloxane)-modified resorcinol–formaldehyde composite xerogel monoliths and the carbonized derivatives. J Sol-Gel Sci Technol 94, 393–405 (2020). https://doi.org/10.1007/s10971-020-05251-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05251-w