Abstract
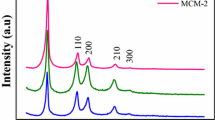
The article deals with the synthesis of mesoporous ordered material MCM-41 from sodium silicate. The series of MCM-41 materials are prepared from industrial sodium silicate using cetyltrimethylammonium bromide (CTAB) as a template and characterized by N2 physisorption and small-angle X-Ray scattering. The MCM-41 sample prepared from the sodium silicate is characterized by high values of specific surface area (1118–1513 m2/g), pore volumes (0.81–0.96 cm3/g), and a well-ordered structure. It was found that the primary assembly of the ordered MCM-41 structure occurs in the solution at room temperature and the MCM-41 may be prepared from the sodium silicate even without hydrothermal treatment. The MCM-41 with a well-ordered structure and a narrow pore size distribution may be synthesized even at a very low CTAB/Si ratio of 0.025–0.125. The MCM-41 prepared from the sodium silicate is stable up to 700 °C and the specific surface area and pore volume were decreased only after the calcination at 800 °C. Thus, the MCM-41 synthesized from the sodium silicate at low CTAB/Si ratio is a promising cheap material for sorption, catalysis and other applications.

Highlights
-
The MCM-41 material with well-ordered structure was prepared from sodium silicate.
-
Decreasing of CTAB/Si ratio from 0.4 to 0.05 leads to narrower pore size distribution.
-
The MCM-41 prepared from sodium silicate is stable up to 700 °C.







Similar content being viewed by others
References
Beck JS, Vartuli JC, Roth WJ, Leonowicz ME, Kresge CT, Schmitt KD, Chu CT-W, Olson DH, Sheppard EW, McCullen SB, Higgins JB, Schlenker JL (1992) A new family of mesoporous molecular sieves prepared with liquid crystal templates. J Am Chem Soc 114(27):10834–10843. https://doi.org/10.1021/ja00053a020
Davis ME (2002) Ordered porous materials for emerging applications. Nature 417(6891):813–821. https://doi.org/10.1038/nature00785
Gibson LT (2014) Mesosilica materials and organic pollutant adsorption: part A removal from air. Chem Soc Rev 43(15):5163–5172. https://doi.org/10.1039/c3cs60096c
Idris SA, Robertson C, Morris MA, Gibson LT (2010) A comparative study of selected sorbents for sampling of aromatic VOCs from indoor air. Anal Methods 2(11):1803–1809. https://doi.org/10.1039/c0ay00418a
Taguchi A, Schüth F (2005) Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater 77(1):1–45. https://doi.org/10.1016/j.micromeso.2004.06.030
Albela B, Bonneviot L (2016) Surface molecular engineering in the confined space of template porous silica. New J Chem 40(5):4115–4131. https://doi.org/10.1039/C5NJ03437J
Hoffmann F, Cornelius M, Morell J, Fröba M (2006) Silica-based mesoporous organic–inorganic hybrid materials. Angew Chem Int Ed 45(20): 3216–3251. https://doi.org/10.1002/anie.200503075
Da’na E (2017) Adsorption of heavy metals on functionalized-mesoporous silica: a review. Microporous and Mesoporous Mater 247:145–157. https://doi.org/10.1016/j.micromeso.2017.03.050
Idris SA, Davidson CM, McManamon C, Morris MA, Anderson P, Gibson LT (2010) Large pore diameter MCM-41 and its application for lead removal from aqueous media. J Hazard Mater 185(2–3):898–904. https://doi.org/10.1016/j.jhazmat.2010.09.105
Gibson LT (2014) Mesosilica materials and organic pollutant adsorption: part B removal from aqueous solution. Chem Soc Rev 43(15):5173–5182. https://doi.org/10.1039/c3cs60095e
Slowing II, Vivero-Escoto JL, Wu C-W, Lin VS-Y (2008) Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv Drug Deliv Rev 60(11):1278–1288. https://doi.org/10.1016/j.addr.2008.03.012
Freitas LB de O, Bravo IJG, Macedo WA de A, de Sousa EMB (2015) Mesoporous silica materials functionalized with folic acid: preparation, characterization and release profile study with methotrexate. J Sol-Gel Sci Technol 43(15):5173–5182. https://doi.org/10.1007/s10971-015-3844-8
Latifi L, Sohrabnezhad S (2018) Influence of pore size and surface area of mesoporous silica materilas (MCM-41 and KIT-6) on the drug loading and release. J Sol-Gel Sci Technol 87(3):626–638. https://doi.org/10.1007/s10971-018-4742-7
Abu Bakar NHH, Bettahar MM, Abu Bakar M, Monteverdi S, Ismail J (2010) Low temperature activation of Pt/Ni supported MCM-41 catalysts for hydrogenation of benzene. J Mol Catal A Chem 333(1–2):11–19. https://doi.org/10.1016/j.molcata.2010.10.007
Mamontov GV, Gorbunova AS, Vyshegorodtseva EV, Zaikovskii VI, Vodyankina OV Selective oxidation of CO in the presence of propylene over Ag/MCM-41 catalyst Catal Today (in press). https://doi.org/10.1016/j.cattod.2018.05.015
Silvestre-Albero J, Serrano-Ruiz JC, Sepúlveda-Escribano A, Rodríguez-Reinoso F (2008) Zn-modified MCM-41 as support for Pt catalysts. Appl Catal A 351(1):16–23. https://doi.org/10.1016/j.apcata.2008.08.021
You, S, Huang, B, Yan, T, Cai, M (2018) A highly efficient heterogeneous palladium-catalyzed carbonylative annulation of 2-aminobenzamides with aryl iodides leading to quinazolinones. J Organomet Chem 875:35–45. https://doi.org/10.1016/j.jorganchem.2018.09.003
Marin ML, Santos-Juanes L, Arques A, Amat AM, Miranda MA (2011) Organic photocatalysts for the oxidation of pollutants and model compounds. Chem Rev 112(3):1710–1750. https://doi.org/10.1021/cr2000543
Linares N, Silvestre-Albero AM, Serrano E, Silvestre-Albero J, García-Martínez J (2014) Mesoporous materials for clean energy technologies. Chem Soc Rev 43(22):7681–7717. https://doi.org/10.1039/c3cs60435g
Kresge CT, Roth WJ (2013) The discovery of mesoporous molecular sieves from the twenty year perspective. Chem Soc Rev 42(9):3663–3670. https://doi.org/10.1039/c3cs60016e
Lin HP, Cheng S, Mou C-Y (1996) Synthesis of thermally stable MCM-41 at ambient temperature. J Chin Chern Soc 43(5):375–378. https://doi.org/10.1002/jccs.199600054
Kirik SD, Belousov OV, Parfenov VA, Vershinina MA (2005) System approach to analysis of the role of the synthesis components and stability of the MCM-41 mesostructured silicate material. Glass Phys and Chem 31(4):439–451. https://doi.org/10.1007/s10720-005-0081-1
Yang G, Deng Y, Ding H, Lin Z, Shao Y, Wang Y (2015) A facile approach to synthesize MCM-41mesoporous materials from iron ore tailing: influence of the synthesis conditions on the structural properties. Appl Clay Sci 111:61–66. https://doi.org/10.1016/j.clay.2015.04.005
Kruk M, Jaroniec M, Sakamoto Y, Terasaki O, Ryoo R, and Ko CH (2000) Determination of pore size and pore wall structure of MCM-41 by using nitrogen adsorption, transmission electron microscopy, and X-ray diffraction. J Phys Chem B 104(2):292–301. https://doi.org/10.1021/jp992718a
Mokhonoana MP, Coville NJ (2010) Synthesis of [Si]-MCM-41 from TEOS and water glass: the water glass-enhanced condensation of TEOS under alkaline conditions. J Sol-Gel Sci Technol 54(1):83–92. https://doi.org/10.1007/s10971-010-2161-5
Halinaa M, Ramesh S, Yarmo MA, Kamarudin RA (2007) Non-hydrothermal synthesis of mesoporous materials using sodium silicate from coal fly ash. Mater Chem Phys 101(2–3):344–351. https://doi.org/10.1016/j.matchemphys.2006.06.007
Boger T, Roesky R, Gläser R, Ernst S, Eigenberger G, Weitkamp J (1997) Influence of the aluminum content on the adsorptive properties of MCM-41. Microporous Mater 8(1–2):79–91. https://doi.org/10.1016/S0927-6513(96)00061-2
Inagaki S, Fukushima Y, Kuroda K (1993) Synthesis of highly ordered mesoporous materials from a layered polysilicate. J Chem Soc Chem Commun (8):680–682. https://doi.org/10.1039/C39930000680
Pauly TR, Petkov V, Liu Y, Billinge SJL, Pinnavaia TJ (2004) Role of framework sodium versus local framework structure in determining the hydrothermal stability of MCM-41 mesostructures. J Am Chem Soc 124(1):97–103. https://doi.org/10.1021/ja0118183
Cai Q, Lin W-Y, Xiao F-S, Pang W-Q, Chen X-H, Zou B-S (1999) The preparation of highly ordered MCM-41 with extremely low surfactant concentration. Microporous Mesoporous Mater 32(1–2):1–15. https://doi.org/10.1016/S1387-1811(99)00082-7
Meynen V, Cool P, Vansant EF (2009) Verified syntheses of mesoporous materials. Microporous Mesoporous Mater 125(3):170–223. https://doi.org/10.1016/j.micromeso.2009.03.046
Landers J, Gor GY, Neimark AV (2013) Density functional theory methods for characterization of porous materials. 437:3–32. https://doi.org/10.1016/j.colsurfa.2013.01.007
Storck S, Bretinger HF, Maier WF (1998) Characterization of micro- and mesoporous solids by physisorption methods and pore-size analysis. Appl Catal A 174(1–2):137–146. https://doi.org/10.1016/S0926-860X(98)00164-1
ALOthman Z (2012) A review: fundamental aspects of silicate mesoporous materials. Materials 5(12):2874–2902. https://doi.org/10.3390/ma5122874
Chen C-Y, Burkett SL, Li H-X, Davis ME (1993) Studies on mesoporous materials II. Synthesis mechanism of MCM-41. Microporous Mater 2(1):27–34. https://doi.org/10.1016/0927-6513(93)80059-4
Sing KS, Everett DH, Haul RA, Moscou L, Pierotti RA, Rouquerol J, Siemicniewska T (1985) Reporting physisorption data for gas solidsystems with special reference to the determination of surface-area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619. https://doi.org/10.1351/pac198557040603
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KSW (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl Chem 87(9–10):1051–1069. https://doi.org/10.1515/pac-2014-1117
Cassiers K, Linssen T, Mathieu M, Benjelloun M, Schrijnemakers K, Van Der Voort P, Cool P, Vansant EF (2002) A detailed study of thermal, hydrothermal, and mechanical stabilities of a wide range of surfactant assembled mesoporous silicas. Chem Mater 14(5):2317–2324. https://doi.org/10.1021/cm0112892
Acknowledgements
This study was supported by “The Tomsk State University Academic D. I. Mendeleev Fund Program (grant No. 8.2.03.2018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vyshegorodtseva, E.V., Larichev, Y. & Mamontov, G.V. The influence of CTAB/Si ratio on the textural properties of MCM-41 prepared from sodium silicate. J Sol-Gel Sci Technol 92, 496–505 (2019). https://doi.org/10.1007/s10971-019-05034-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-019-05034-y