Abstract
In this work, we study the Y2O3:Er3+-Gd3+ and Y2O3:Eu3+-Gd3+ nanocrystals using statistical tools in order to quantify the effect of dopant concentration, crystallite size, induced strain, and lattice parameters on luminescent parameters such as intensity and color emission. Samples were synthesized using the sol–gel method. In the case of emission properties of Y2O3:Er3+-Gd3+, the most important factor is Erbium concentration, while the energy transfer between Gadolinium-Erbium is statistically insignificant, compared to the dynamics between Erbium–Erbium ions. On the other hand, the Y2O3:Eu3+-Gd3+ emission intensity depends on the dopant concentration: an increase of Gadolinium ions increases the emission intensity, and the quadratic effect of the Eu3+ ions reduce the emission intensity. However, the color coordinate is not so influenced by the dopant concentration; in the case of Y2O3:Er3+-Gd3+ samples, the color coordinate depends only on Er3+ concentration.

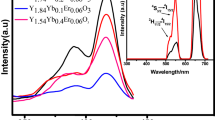
Stokes luminescence process of Gd3+, Eu3+, Er3+ ions, and charge‐transfer bands responsible for the visible emission.













Similar content being viewed by others
References
Thejo Kalyani N, Dhoble SJ (2012) Organic light emitting diodes: energy saving lighting technology—a review Renew Sustain Energy Rev 16(5):2696–2723
Rangari VV, Dhoble SJ (2015) Synthesis and photoluminescence studies of Ba(Gd, Ln) B9O16:Eu3+ (Ln=La,Y) phosphors for n-UV LED lighting and display devices J Rare Earths 33(2):140–147
Patra A, Friend CS, Kapoor R, Prasad PN (2002) Upconversion in Er3+:ZrO2 Nanocrystals. J Phys Chem B 106:1909–1912
De la Rosa E, Diaz-Torres LA, Salas P, Rodríguez RA (2005) Visible light emission under UV and IR excitation of rare earth doped ZrO2 nanophosphor Opt Mater 27(7):1320–1325
Lupei A, Lupei V, Hau S (2017) Vibronics in optical spectra of Yb3+ and Ce3+ in YAG and Y2O3 ceramics. Opt Mater 63:143–152
Rodríguez VD, Tikhomirov VK, Méndez-Ramos J, Yanes AC, Moshchalkov VV (2010) Towards broad range and highly efficient down-conversion of solar spectrum by Er3+–Yb3+ co-doped nano-structured glass-ceramics Sol Energy Mater Sol Cells 64(10):1612–1617
Aarts L, van der Ende BM, Meijerink A (2009) Downconversion for solar cells in NaY F4:Er; Yb J Appl Phys 106(2):23522
Camposeco-Negrete C (2013) Optimization of cutting parameters for minimizing energy consumption in turning of AISI 6061 T6 using Taguchi methodology and ANOVA. J Clean Prod 53:195–203
Feng J, Zhang J, Zhang J, He Y, Zhang R, Chen C, Liu G (2017) Enhanced methane production of vinegar residue by response surface methodology (RSM). AMB Express 7:89
Alhaji A, Razavi RS, Ghasemi A, Loghman-Estarki MR (2017) Modification of Pechini sol–gel process for the synthesis of MgO-Y2O3 composite nanopowder using sucrose-mediated technique Ceram Int 43(2):2541–2548
Huízar-Félix AM, Hernández T, de la Parra S, Ibarra J, Kharisov B (2012) Sol-gel based Pechini method synthesis and characterization of Sm1−xCaxFeO3 perovskite 0.1 × 0.5. Powder Technol 229:290–293
Dimesso L, Klein L, Aparicio M, Jitianu A (2016) Pechini processes: an alternate approach of the sol–gel method, preparation, properties, and applications. In: Handbook of sol-gel science and technology. Springer International Publishing, Switzerland, pp 1–22
Laberty-Robert C, Ansart F, Castillo S, Richard G (2002) Synthesis of YSZ powders by the sol-gel method: Surfactant effects on the morphology Solid State Sci 4(8):1053–1059
Xie Y, Wang G, Xiao LJ, Li WZ, Chen YJ, Zhang ZG, Wang YJ (2013) Effects of surfactants on particle size and luminescence properties of Ca1.98MgSiO5:Eu2+ 0.02 phosphor prepared by sol-gel method. Adv Mater Res 634:2454–2457
Kriz DA, He J, Pahalagedara M, Suib SL (2017) Response to comments on the application of the Scherrer equation in “Copper aluminum mixed oxide (CuAl MO) catalyst: a green approach for the one-pot synthesis of imines under solvent-free conditions”, by Suib et al. ((2016) Appl Catal B Environ 188:227–234. https://doi.org/10.1016/j.apcatb.2016.02.007). Article type: Correspondence 202:704–705
Lakowicz JR (2006) Principles of fluorescence spectroscopy principles of fluorescence spectroscopy, 3rd edn. Springer-Verlag, US, pp 1–60.
Thoma RE, Insley H, Hebert GM (1966) The sodium fluoride-lanthanide trifluoride systems Inorg Chem 5(7):1222–1229
Wegh RT, Meijerink A, Lamminmäki R-J, Jorma H (2000) Extending Dieke’s diagram. J Lumin 8:1002–1004
Meza O, Villabona-Leal EG, Diaz-Torres LA, Desirena H, Rodríguez-López JL, Pérez E (2014) Luminescence concentration quenching mechanism in Gd2O3:Eu3+ J Phys Chem A 118(8):1390–1396
Montgomery DC (2006) Design and analysis of experiments. John Wiley & Sons, US, pp 1–30
Solé JG, Bausá LE, Jaque D (2005) An introduction to the optical spectroscopy of inorganic solids. John Wiley & Sons, US, Ltd., pp 1–38
Meza O, Diaz-Torres La, Salas P, De la Rosa E, Solis D (2010) Color tunability of the upconversion emission in Er–Yb doped the wide band gap nanophosphors ZrO2 and Y2O3. Mater Sci Eng: B 174:177–181
Ohta N, Robertson A (2005) Colorimetry: fundamentals and applications. Wiley, US, p 350
Gamelin DR, Gudel HU (2001) Transition metal and rare earth compounds: excited states, transitions, interactions II, upconversion processes in transition metal and rare earth metal systems. Springer, Berlin Heidelberg, pp 1–56
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Highlights
-
1.
Colorimetric properties as a function of the crystal structure, and dopant the effects are estimated.
-
2.
It is possible to control the color coordinate by varying only the Er3+ concentration.
-
3.
Gd3+ increases the luminescence intensity when it is combined with Eu3+ ions; however, with Er3+ ions, the combination has no effect.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Rodríguez, S.V., Villabona-Leal, E.G., Mixteco-Sánchez, J.C. et al. Study of visible light emission under UV excitation in Y2O3:Er3+-Gd3+ and Y2O3:Eu3+-Gd3+ nanocrystals. J Sol-Gel Sci Technol 86, 782–794 (2018). https://doi.org/10.1007/s10971-018-4661-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-018-4661-7