Abstract
A potential UV-excitable green-emitting erbium-doped α-Na3Y(VO4)2 nanocrystalline phosphor has been successfully synthesized via facile citrate-based solution combustion method. The structural characterization of the sample was carried out using X-ray diffraction, TEM, HRTEM, SAED, EDS and FTIR analysis. The phosphor exhibits green emission at 523 nm and 552 nm, which correspond to 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 transitions of Er3+, respectively. The energy transfer mechanism among Er3+ ions and the concentration mechanism have been studied. The optimal doping concentration of Er3+ in the α-Na3Y(VO4)2 is found to be 0.025 mol. The non-radiative interaction among Er3+ is attributed to the dipole–quadrupole interaction. The quantum yield is found to be 7.25%, and chromaticity coordinates for the optimal concentration are found to be (0.2571, 0.6707) which are close to National Television System Committee standards for green (0.21, 0.71). The lifetime of erbium emission is found to be in microsecond range. The photoluminescence studies reveal that the newly synthesized Er3+-doped α-Na3Y(VO4)2 nanocrystals would be a good choice as green emitter to be combined with previously investigated blue- and red-emitting Tm3+- and Sm3+-doped α-Na3Y(VO4)2 nanocrystals for realizing RGB phosphor.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Phosphor-converted (pc) WLED is rapidly replacing the conventional incandescent and fluorescence lamp in the field of general lighting and display backlight, owing to their surpassing benefits such as low energy consumption, low heat output, small footprints, spectral tunability, long lifetime as well as efficient conversion of electric power into optical power [1]. Coating of luminescent materials onto the surface of the UV/blue LED chip is the common method employed in improvising phosphor-converted WLED. This can be achieved by the combination of a blue LED with a yellow phosphor (YAG:Ce3+) or a combination of UV-LED with tricolor red, green and blue (RGB) phosphors or a combination of UV-LED with single-phased white-emitting phosphors [2]. Among them, combination of UV-LED with single-phased tricolor-based white-emitting phosphors is significantly employed in WLED industry owing to their simplicity in operation, good luminous efficiency, low color correlated temperature (CCT), high color rendering index (CRI) and low manufacturing cost. A potential single-phased tricolor-based white phosphor with good absorption in the UV region, low correlated color temperature (CCT) and high color rendering index (CRI) is a matter of prime importance.
Among various rare earth-doped inorganic phosphors oxides, rare earth vanadates are an important class of luminescent materials. In addition to the good chemical stability, moisture resistance, inertness and eco-friendliness, rare earth-doped vanadates are attracting much attention due to their capacity to transfer energy from charge transfer band of vanadate to almost all luminescent rare earth ions efficiently ensuring intense characteristic emission in the entire visible range [3]. Rare earth ions are characterized by a partially filled 4f which is well screened by the 5s2 and 5p6 orbitals, yielding sharp emission lines especially in the visible region [4]. It is reported that yttrium vanadate single crystal can serve as laser host material [5] and Eu3+-doped yttrium vanadate has been used as commercial red phosphor in fluorescent lamp and cathode ray tube for last two decades [6, 7]. Rare earth-doped double vanadates of the form A3B(VO4)2 (A denotes alkali metal like Na, K, Li and B represents La, Y, Gd) have attained significant attention in lighting applications [8, 9]. α-Na3Y(VO4)2 has been selected as host lattice in this work, owing to their lower symmetry (C2v) occupied by Y3+ ions in α-Na3Y(VO4)2 compared to the D2d symmetry of Y3+ ions in tetragonal YVO4 [10]. We have already reported blue-emitting thulium-doped and reddish-orange-emitting samarium-doped α-Na3Y(VO4)2 nanocrystals in which intense luminescence emission is achieved via host sensitization [11, 12]. The current work is a follow-up in search of a green-emitting rare earth-doped α-Na3Y(VO4)2 in order to realize phosphors suitable for white light applications. Even though terbium is the commonly used green-emitting ion, terbium-doped α-Na3Y(VO4)2 is not a proper choice to realize the green component in RGB phosphor since there is no significant energy transfer from vanadate band to terbium ion [13]. Therefore, the search for new green-emitting vanadate phosphor leads to the investigation on photoluminescence studies of Er3+-doped α-Na3Y(VO4)2 nanocrystals. In this work, synthesis and photoluminescence characteristics of green-emitting erbium-doped α-Na3Y(VO4)2 nanocrystalline phosphor are reported.
2 Experimental methods
2.1 Synthesis
Solution combustion method with citric acid as the fuel was employed for the synthesis of erbium-doped sodium yttrium vanadate [α-Na3Y1−xErx(VO4)2] nanocrystals. A set of samples with varying erbium concentrations corresponding to x = 0, 0.01, 0.015, 0.02, 0.025, 0.03, 0.04, 0.05 were prepared. Sodium hydroxide (NaOH), ammonium vanadate (NH4VO3), ammonia solution, citric acid monohydrate (C6H8O7·H2O), high purity erbium (III) nitrate pentahydrate (Er(NO3)3·5H2O (99.99%)) and yttrium (III) nitrate hexahydrate (Y(NO3)3·6H2O (99.9%)) were used as the raw materials. All the chemicals used were of analytical grade. The chemical reaction leading to the formation of erbium-doped sodium yttrium vanadate is
Orthovanadate solution was prepared by mixing 10 ml aqueous solutions of 0.2 M NH4VO3 and 0.3 M NaOH under stirring followed by heating at 70 °C for 1 h. 0.1 M aqueous solutions of Y(NO3)3 and Er(NO3)3 were mixed in the required stoichiometric ratio and added with citric acid keeping the ratio of total metal cations to citric acid as 1:4. This solution was then added drop wise into orthovanadate solution under stirring. The resulted yellowish orange solution was further heated at 80 °C for 1 h in a closed beaker and the color changed into blue. This solution was then allowed to evaporate by boiling at 120 °C for 1 h and was transferred into a muffle furnace and sintered at 500 °C for 2 h at the rate 300 °C/h and then allowed to cool. Fine powder of erbium-doped α-Na3Y(VO4)2 nanocrystals thus formed were used for further analysis.
2.2 Characterization
X-ray powder diffraction patterns of the synthesized erbium-doped sodium yttrium vanadate samples were recorded in the 2θ range 10°–60° using Rigaku RU-200B X–ray diffractometer. Fourier transform infrared (FTIR) spectra of the samples were traced by a Shimadzu IR Prestige 20 spectrometer. Energy-dispersive X-ray spectrometry was employed to study the chemical composition of the sample using Carl Zeiss EVO 18 Secondary Electron Microscope with EDS. Transmission Electron Microscopic analysis was carried out using a JEOL JEM 2100 transmission electron microscope. The absorption spectra of the sample were recorded using Perkin Elmer Lambda 25 UV–Vis spectrometer in the wavelength range 200–800 nm. Photoluminescence excitation, emission and decay analysis were carried out on a Horiba Jobin–Yvon Fluoromax-4 spectrofluorometer.
3 Results and discussion
3.1 X-ray diffraction analysis
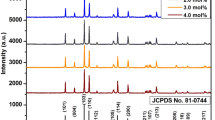
The X-ray diffraction patterns of erbium-doped sodium yttrium vanadate nanocrystals with varying erbium doping concentration are shown in Fig. 1. The well-defined diffraction peaks in the XRD profile ascertain the highly crystalline nature of prepared samples. The diffraction peaks of all the prepared samples match with that of pure α-Na3Y(VO4)2 with monoclinic phase as per the standard data in ICDD file No. 55-0797. Table 1 represents the computed lattice parameters and the cell volume of the nanocrystals using the unit cell win software.
Close resemblance in the lattice parameters among various samples is ascribed due to the proximity in the ionic radii of yttrium (0.9 Å) and erbium (0.89 Å) with similar valences as well as due to the successful incorporation of erbium into the host lattice. Scherrer equation, \(D = \frac{0.9 \lambda }{{\beta \text{Cos} \theta }}\), has been employed to estimate average crystallite size of the sample, where D is the average crystallite size, λ is the X-ray wavelength (1.548 Å), θ is the diffraction angle, and β is the full width half maximum (in radians) of the peak [14]. The estimated crystallite size of samples with various erbium concentrations is found to be in the range 22–33 nm and is also tabulated in Table 1.
α-Na3Y(VO4)2 is found to be iso-structural with monoclinic α-Na3Er(VO4)2 with slightly distorted glasserite lattice with space group P21/n, No.14 and Z = 2. Isolated VO43− tetrahedra, YO6 and NaO6 octahedron constitute the crystal structure of α-Na3Y(VO4)2. The site symmetry of Y3+ and Er3+ is C2v, whereas VO43− possesses the point symmetry of C1 [11]. The crystal structure has an effect on the luminescence properties of rare earth-doped α-Na3Y(VO4)2, and it is identified as an excellent phosphor for solid-state lighting applications.
3.2 Energy-dispersive X-ray analysis
The chemical purity of the sample is further verified by Energy-dispersive X-ray analysis, and the EDS spectrum of the sample α-Na3Y0.975Er0.025(VO4)2 is shown in Fig. 2. The peaks present in the spectrum corresponding to sodium (Na), yttrium (Y), erbium (Er), vanadium (V), oxygen (O) validate the chemical composition and chemical purity of the samples.
3.3 Microstructural analysis
Figure 3 illustrates the microstructural features of α-Na3Y0.975Er0.025(VO4)2 nanocrystals—a representative among the synthesized samples. TEM image shown in Fig. 3a indicates that the nanocrystals are of spherical shape and are well dispersed. The statistical particle distribution curve is plotted as shown in Fig. 3b, and the particle size is found to be in the range 10–50 nm. The formation of spherical nanocrystals is due to the presence of excess citric acid in confirmation with the previous reports [15].
Clear fringes in the HRTEM image corroborate the high crystalline nature of the α-Na3Y0.975Er0.025(VO4)2 sample as shown in Fig. 3c. The presence of the (121) crystal planes corresponding to spacing d = 3.202 Å is recognized in the HRTEM image which is consistent with the XRD data. Selected area electron diffraction (SAED) image of α-Na3Y0.975Er0.025(VO4)2 sample is displayed in Fig. 3d. Well-defined rings patterns are attributed to the polycrystalline nature of the sample. The planes (121), (031) and (222) which are associated with d = 3.19, 2.80 and 1.951 Å, respectively, are identified and are in close agreement with the standard ICDD of α-Na3Y(VO4)2.
3.4 Fourier transform infrared (FTIR) spectroscopy analysis
The vibrational modes in α-Na3Y1−xErx(VO4)2 where x = 0, 0.01, 0.02, 0.025, 0.030, 0.04, 0.05 were identified from the FTIR spectrum displayed in Fig. 4. Tetrahedral VO43− ion possesses Td symmetry and has four normal modes of which only two are IR active. When the vanadate group becomes the part of monoclinic α-Na3Y(VO4)2 lattice, the VO43− group gets slightly distorted and the symmetry shifts to a lower one (C1) and all the fundamental modes become both IR and Raman active [16]. The bands below 1000 cm−1 are assigned due to internal vibration of the VO43− ions. Strong bands observed at 740 cm−1 and 960 cm−1 are identified as ν3 mode of VO43−. The band at 410 cm−1 is attributed to ν2 and ν4 modes and that at 905 cm−1 as due to ν1 mode of VO43−. Feeble peaks at 3343 cm−1, 2301 cm−1 and 1449 cm−1 are due to the stretching and bending modes of OH vibrations and are less significant [17].
4 Optical characterization
4.1 UV–Vis absorption analysis
UV–Vis absorption spectra of erbium-doped α-Na3Y(VO4)2 nanocrystals were recorded in order to have a better insight into the photoluminescence characteristics of the prepared phosphor. The absorption spectra in the range 200–800 nm for the samples with varying erbium concentration are depicted in Fig. 5. A strong and broad absorption band in the UV region is present in all the spectra attributing to the overlapped O2− to Er3+ and O2− to V5+ metal–ligand charge transfer transitions [18]. In the longer wavelength region (above 350 nm), narrow absorption peaks due to f–f transitions of Er3+ are expected, but in the present case only a small peak is observed at 522 nm which is attributed to the transition from the ground state of erbium 4I15/2 to 2H11/2. No other peaks are detected in the present case, may be due to the low doping content of Er3+ and pronounced intensity of host absorption band. The intense and broadband in the UV region is a promising pathway for energy transfer from vanadate host to erbium ions and offers flexibility in the choice of excitation wavelength in UV region.
4.2 Photoluminescence studies
The excitation spectra of α-Na3Y1−xErx(VO4)2 nanocrystals with a range of erbium concentrations corresponding to x = 0.01, 0.02, 0.025, 0.030, 0.04, 0.05 were recorded in the range 230–420 nm monitoring the 552 nm emission of Er3+ and are displayed in Fig. 6. The broadband peaked around 310 nm arises due to the charge transfer transitions [19]. The sharp peaks at 366 nm, 380 nm and 407 nm are assigned, respectively, due to 4I15/2 → 4G9/2, 4I15/2 → 4G11/2 and 4I15/2 → 4H9/2 transitions of Er3+ ion [20]. The appearance of strong and extended excitation peak in the UV range on monitoring the emission of Er3+ ascertains the sensitization of erbium via host.
Figure 7 illustrates the photoluminescence emission spectra of α-Na3Y1−xErx(VO4)2 where x = 0.01, 0.02, 0.025, 0.030, 0.04, 0.05 under 310 nm host excitation. Green emission centered at 523 nm and 552 nm was detected and attributed to the transitions from 2H11/2 and 4S3/2 levels to the 4I15/2 ground state, respectively. Violet emission around 408 nm (2H9/2 → 4I15/2) and red emission around 660 nm (4F9/2 → 4I15/2) were hardly visible revealing the efficient non-radiative relaxation from 2H9/2 and 4F9/2 [20]. The prevalent green emission in the emission spectra as depicted in Fig. 8 brings out α-Na3Y1−xErx(VO4)2 nanocrystal as an excellent green phosphor with better chromaticity for white light generation.
Various radiative and non-radiative pathways involved in the photoluminescence mechanism of Er3+ in α-Na3Y(VO4)2 is picturized in the schematic energy level diagram as shown in Fig. 9. Vanadate ion in 1A1 ground state could be excited to 1T2 state upon excitation at 310 nm and then non-radiatively relaxed to 1T1 state. The 1T1 excited state energy of vanadate is almost the same as that of 4G7/2 excited state of Er3+ resulting in non-radiative resonant energy transfer from vanadate to Er3+ ions. The 4G7/2 excited state of Er3+ gets depopulated non-radiatively into 2H11/2 and 4S3/2 emitting state of Er3+ ions which trigger the green emission around 523 nm and 552 nm, respectively. The possible non-radiative cross-relaxation channels responsible for concentration quenching are 4I9/2 + 4G11/2 → 2H11/2 + 2H11/2 or 4S3/2 and 4I15/2 + 4G11/2 → 4I13/2 + 2H11/2 or 4S3/2 and are represented as CR1 and CR2, respectively, in Fig. 9 [21].
Photoluminescence emission spectra of the samples (Fig. 7) also show that the intensity of green emission goes on increasing with the increase in doping concentration Er3+ up to the optimal concentration x = 0.025 mol and then decreases in tune with the trend observed in the excitation spectra. The plot of intensity of 552 nm emission versus Er3+ concentration when excited at 310 nm is displayed in Fig. 10. A sudden fall in luminescence intensity on increasing Er3+ content beyond x = 0.025 mol arises from the concentration quenching effects due to non-radiative cross-relaxation path and the energy transfer among Er3+ ions until energy sink in the lattice is reached.
The type of non-radiative energy transfer among Er3+ ions that leads to concentration quenching strongly depends on the critical distance (Rc) between adjacent Er3+ ions. The critical distance (Rc) between adjacent Er3+ ions as provided by the following Blasse equation is
where V is the unit cell volume, N is the number of Er3+ ions in a unit cell, \(x_{c}\) is the optimal concentration, and the critical distance is calculated to be about 24.55 Å [22]. If the critical distance (Rc) between adjacent Er3+ ions is less than 5 Å, the interaction is mainly exchange interaction whereas the critical distance (Rc) is greater than 5 Å, multipolar interaction is responsible for concentration quenching. The type of multipolar interaction can be identified using Dexter’s formula [23].
where I is the intensity corresponding to the doping concentration (C). Q represents the type of multipolar interaction and Q = 6, 8, 10 correspond to the dipole–dipole, dipole–quadrupole and quadrupole–quadrupole interactions, respectively. log (I/C) − log (C) curve is plotted for different α-Na3Y1−xErx(VO4)2 nanocrystals (x = 0.01, 0.02, 0.025, 0.030, 0.04, 0.05) for green emission (552 nm) excited at 310 nm and is found to be linear as depicted in Fig. 11. The value of Q is calculated as 8.57 which indicates that the non-radiative energy transfer among Er3+ ions is caused by dipole–quadrupole interaction. The quantum yield of the α-Na3Y1−xErx(VO4)2 nanocrystals with x = 0.025 is found to be 7.25% and comparable with earlier reported cases [24].
4.3 Luminescence decay analysis
Figure 12a displays the decay curves of α-Na3Y1−xErx(VO4)2 nanocrystals (x = 0, 0.01, 0.025 and 0.05) monitoring the host emission at 438 nm on exciting at 310 nm. All the decay curves of host emission are found to be double exponential, and the luminescence emission persists up to 80 μs. The short lifetimes are found to be 0.616, 0.554 and 0.553 μs, and the long lifetimes are 12.393, 11.571, 10.99 μs for erbium concentrations x = 0.01, 0.025 and 0.05, respectively. This double exponential decay nature indicates energy transfer from vanadate to erbium. The average lifetime of the host emission is calculated using the formula \(t_{\text{avg}} = \frac{{A_{1} t_{1}^{2} + A_{2} t_{2}^{2} }}{{A_{1} t_{1} + A_{2} t_{2} }} ,\) where \(t_{1}\) and \(t_{2}\) are the lifetime values and \(A_{1 }\) and \(A_{2 }\) are the corresponding weight factors. The calculated average lifetime values are 10.128, 9.067 and 8.663 μs corresponding to erbium concentrations x = 0.01, 0.025 and 0.05, respectively.
The decay curves of 552 nm erbium emission monitored upon 310 nm excitation are shown in Fig. 12b. The nature of the decay curves seems to be multi-exponential by appearance on account of the very slow decay in the beginning of the curve. But the decay curves are best fitted to single exponential in nature, since the slow decay in the beginning of the curve is less significant considering the general nature of the decay process. The lifetime values obtained by the single exponential fitting are 11.045, 10.519 and 10.466 μs corresponding to erbium concentrations x = 0.01, 0.025 and 0.05, respectively, matching with similar reports [25]. The slight decrease in the lifetime values is due to the luminescence quenching due to the interaction among the Er3+ ions. The energy transfer efficiency from vanadate to erbium in α-Na3Y1−xErx(VO4)2 nanocrystals (x = 0.01, 0.025 and 0.05) was calculated as 40.725, 53.285 and 56.385% using the equation \(\eta_{\text{ET}} = 1 - \frac{{\tau_{s} }}{{ \tau_{0} }}\), where τ0 and τs refer to the lifetime of vanadate emission without the activator Er3+ and with Er3+ ions, respectively, on host excitation 310 nm.
4.4 Colorimetric analysis
In order to have a better understanding on the quality of emitting color of the phosphor which is very significant in various lighting applications, CIE colorimetric studies can be employed. The Commission Internationale de l’Éclairage chromaticity diagram of α-Na3Y1−xErx(VO4)2 (where x = 0.01, 0.02, 0.025, 0.030, 0.04, 0.05) nanocrystalline phosphors under 310 nm excitation was plotted using the color calculator software and is exhibited in Fig. 13. The corresponding digital photograph of α-Na3Y1−xErx(VO4)2 (x = 0.025) is shown in the inset of Fig. 13. The chromaticity coordinates of the samples were evaluated and are tabulated in Table 2 [26]. The CIE chromaticity coordinates of α-Na3Y0.975Er0.025(VO4)2 (0.25, 0.67) are found to be very close to National Television System Committee (NTSC) standard green (0.21, 0.71) as well as European broadcasting union (EBU) standard green (0.29, 0.60) with high color purity (84%).
4.5 Conclusion
Erbium-activated α-Na3Y(VO4)2 was synthesized via fast and facile low temperature citrate-based solution combustion method. X-ray diffraction studies confirmed the monoclinic phase of the sample and the chemical composition is validated by EDS analysis. FTIR studies also confirmed the formation of α-Na3Y(VO4)2 phase by observing the characteristic peaks. The highly crystalline nature and uniform spherical morphology of the sample were revealed by TEM analysis. Efficient energy transfer from host to Er3+ is proposed on the basis of UV–Vis absorption and photoluminescence excitation studies. Under 310 nm host excitation, α-Na3Y1−xErx(VO4)2 nanocrystals exhibit intense green emission around 523 nm and 552 nm corresponding to the transitions 2H11/2 → 4I15/2 and 4S3/2 → 4I15/2 of Er3+, respectively. The quantum yield of the sample is found to be 7.25%, and energy transfer efficiency is calculated as 56% for optimum concentration of erbium. The lifetime of erbium emission in α-Na3Y1−xErx(VO4)2 is found to be in the microsecond range. From the CIE chromaticity diagram, the potential of α-Na3Y1−xErx(VO4)2 nanocrystals as a green phosphor with high color purity (84%) is ensured. The proximity of the chromaticity coordinate values of the above sample with the NTSC and EBU standards makes it suitable for developing single wavelength excitable phosphor-converted white LED applications.
References
Smet PF, Joos JJ (2017) White light-emitting diodes: stabilizing colour and intensity. Nat Mater 16:500–501. https://doi.org/10.1038/nmat4901
Vishwakarma AK, Jha K, Jayasimhadri M, Sivaiah B, Gahtori B, Haranath D (2015) Emerging cool white light emission from Dy3+ doped single phase alkaline earth niobate phosphors for indoor lighting applications. Dalt Trans 44:17166–17174. https://doi.org/10.1039/c5dt02436f
Huang X, Guo H (2018) Synthesis and photoluminescence properties of Eu3+-activated LiCa3ZnV3O12 white-emitting phosphors. RSC Adv 8:17132–17138. https://doi.org/10.1039/c8ra03075h
Blasse G, Grabmaier BC, Blasse G, Grabmaier BC (2011) A general introduction to luminescent materials. Lumin Mater. https://doi.org/10.1007/978-3-642-79017-1_1
Morrison AD (1973) Note on inclusions in yttrium vanadate crystals. J Mater Sci 8:1666–1669. https://doi.org/10.1007/BF00754903
Palilla FC (1964) A new, highly efficient red-emitting cathodoluminescent phosphor (YVO4:Eu) for color television. Appl Phys Lett 5:118–120. https://doi.org/10.1063/1.1723611
Levine AK, Palilla FC (1965) YVO4: Eu-A 10 New red-emitting phosphor for color television. Trans N Y Acad Sci 27:517–529. https://doi.org/10.1111/j.2164-0947.1965.tb02210.x
David ADJ, Muhammad GS, Sivakumar V (2016) Synthesis and photoluminescence properties of Sm3+substituted glaserite-type orthovanadates K3Y[VO4]2 with monoclinic structure. J Lumin 177:104–110. https://doi.org/10.1016/j.jlumin.2016.04.025
Hussain SK, Giang TTH, Yu JS (2018) UV excitation band induced novel Na3Gd(VO4)2:RE3+ (RE3+ = Eu3+ or Dy3+ or Sm3+) double vanadate phosphors for solid-state lightning applications. J Alloys Compd 739:218–226. https://doi.org/10.1016/j.jallcom.2017.12.200
Miller SA, Caspers HH, Rast HE (1968) Lattice vibrations of Yttrium Vanadate. Phys Rev 168:964–969. https://doi.org/10.1103/PhysRev.168.964
Jacob LA, Sisira S, Mani KP, Thomas K, Alexander D, Biju PR, Unnikrishnan NV, Joseph C (2020) High purity blue photoluminescence in thulium activated α-Na3Y(VO4)2 nanocrystals via host sensitization. J Lumin 223:117169. https://doi.org/10.1016/j.jlumin.2020.117169
Jacob LA, Sisira S, Thomas K, Alexander D, Biju PR, Unnikrishnan NV, Joseph C (2019) A reddish-orange emitting samarium doped α-Na3Y(VO4)2 nanocrystals for single phased UV excitable white light applications. J Solid State Chem 280:120998. https://doi.org/10.1016/j.jssc.2019.120998
Huang P, Chen D, Wang Y (2011) Host-sensitized multicolor tunable luminescence of lanthanide ion doped one-dimensional YVO4 nano-crystals. J Alloys Compd 509:3375–3381. https://doi.org/10.1016/j.jallcom.2010.12.069
Patterson AL (1939) The scherrer formula for X-ray particle size determination. Phys Rev 56:978–982. https://doi.org/10.1103/PhysRev.56.978
Du G, Kan X, Han Y, Sun Z, Guo W (2012) Citric acid assisted hydrothermal synthesis of microparticles of ErPO4:Dy3+. Mater Lett 79:55–57. https://doi.org/10.1016/j.matlet.2012.03.086
Sobczyk M (2013) Optical properties of α-Nd3+: Na3Y(VO 4)2 single crystals-Potential laser materials. Opt Mater (Amst) 35:852–859. https://doi.org/10.1016/j.optmat.2012.10.043
Rao BV, Jang K, Sueb H, Yi S, Jeong J (2010) Synthesis and photoluminescence characterization of RE3+ (= Eu3+, Dy3+) -activated Ca3La(VO4)3 phosphors for white light-emitting diodes. J Alloys Compd 496:251–255. https://doi.org/10.1016/j.jallcom.2009.12.175
Zhang Q, Hu Y, Ju G, Zhang S, Xue F (2017) Photoluminescence of a novel Na3Y(VO4)2:Eu3+ red phosphor for near ultraviolet light emitting diodes application. J Mater Sci: Mater Electron 28:2529–2537. https://doi.org/10.1007/s10854-016-5827-0
Kimani MM, Kolis JW (2014) Synthesis and luminescence studies of a novel white Dy:K3Y(VO4)2 and yellow emitting phosphor Dy,Bi:K3Y(VO4)2 with potential application in white light emitting diodes. J Lumin 145:492–497. https://doi.org/10.1016/j.jlumin.2013.07.054
Yan SA, Wang JW, Chang YS, Hwang WS, Chang YH (2011) Synthesis and luminescence properties of Ln3+ (Ln3+ = Er3+, Sm3+)-doped barium lanthanum tungstate BaLa2WO7 phosphors. Opt Mater (Amst) 34:147–151. https://doi.org/10.1016/j.optmat.2011.07.028
Song F, Zhang G, Shang M, Tan H, Yang J, Meng F (2001) Three- photon phenomena in the upconversion luminescence of erbium–ytterbium codoped phosphate glass. Appl Phys Lett 79:1748. https://doi.org/10.1063/1.1404996
Blasse G (1968) Energy transfer in oxidic phosphors. Phys Lett A 28:444–445. https://doi.org/10.1016/0375-9601(68)90486-6
Dexter DL (1953) A theory of sensitized luminescence in solids. J Chem Phys 21:836–850. https://doi.org/10.1063/1.1699044
Pokhrel M, Kumar GA, Ma C, Brik M, Langloss B, Shanton I, Therien MJ, Sardar D, Mao Y (2015) Electronic and optical properties of Er: doped Y2O2S phosphors. J Mater Chem C 3(43):11486–11496. https://doi.org/10.1039/C5TC02665B
Sreeja E, Mohan RP, Gopi S, Joseph C, Unnikrishnan NV, Biju PR (2019) Structural and photoluminescence properties of UV-excited Er3+ doped Ba2CaWO6 yellowish-green phosphors. Phys B Condens Matter 555:284–291. https://doi.org/10.1016/j.physb.2018.11.020
Smith T, Guild J (1931) The C.I.E. colorimetric standards and their use. Trans Opt Soc 33:73–134
Acknowledgements
Linju Ann Jacob, first author of this research paper, is extremely grateful to UGC, Govt. of India for providing financial support through faculty development program). Mrs. Sisira S, Mrs. Kukku Thomas and Mrs. Dinu Alexander are thankful for the award of research fellowships to CSIR, Govt. of India (JRF fellowship), UGC, Govt. of India (MANF fellowship) and UGC, Govt. of India (RFSMS fellowship), respectively. The authors are thankful to Department of Science and Technology, Govt. of India and for the support through the programs of SAP-DRS (No. F.530/12/DRS/2009 (SAP-1)) and DST-PURSE (SR/417&418/2017)), respectively. A special thanks to MoU-DAE-BRNS Project (No. 2009/34/36/BRNS/3174) and to department of Physics, St. Thomas College, Palai, India, for extending their experimental facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jacob, L.A., Sisira, S., Mani, K.P. et al. A new potential green-emitting erbium-activated α-Na3Y(VO4)2 nanocrystals for UV-excitable single-phase pc-WLED applications. SN Appl. Sci. 2, 1076 (2020). https://doi.org/10.1007/s42452-020-2873-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s42452-020-2873-2