Abstract
A novel simple synthetic procedure for improving cell efficiency and reducing the production cost of TiO2 dye-sensitized solar cells (DSSCs) by modification and optimization of homemade formulated paste is reported. This is achieved in terms of morphological manipulation of deposited monolayer TiO2 films by controlling three processing parameters of paste formulation. These parameters are tailored to obtain a paste with proper viscosity suitable for spin-coating technique and to achieve uniform, homogeneous, and crack-free films with good connections between TiO2 grains and porous structure. Photovoltaic measurements show that TiO2 monolayer DSSC fabricated from the optimized paste formulation (i.e., T7B5G12) has the highest power conversion efficiency (PCE) and short-circuit current (J SC) of 5.36 ± 0.04 % and 14.29 ± 0.02 mA/cm2, respectively. The double-layer DSSCs are also fabricated to enhance electron transport rate and light scattering of the cells. These cells are composed of T7B5G12 film as the under-layer and mixtures of various morphologies (i.e., nanoparticles, regular nanowires, corn-like nanowires, and microparticles) as the over-layers. An enhancement of PCE and J SC up to 7.05 ± 0.024 % and 16.67 ± 0.04 mA/cm2, respectively, is achieved for double-layer DSSC containing a mixture of nanoparticles and corn-like nanowires, as the over-layer. The photovoltaic improvement of double-layer cells is related to tailoring the light scattering, photogenerated charge carriers, and dye-loading parameters.
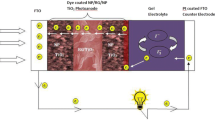
Graphical Abstract











Similar content being viewed by others
References
O’Regan B, Gratzel M (1991) A low-cost, high-efficiency solar cell based on dye sensitized colloidal TiO2 films. Nature 353:737–739
Lewis NS (2007) Toward cost-effective solar energy use. Science 315:798–801
Kim S, Kim D, Choi H, Kang MS, Song K, Kang SO, Ko J (2008) Enhanced photovoltaic performance and long-term stability of quasi-solid-state dye-sensitized solar cells via molecular engineering. Chem Commun 40:4951–4953
Caramori S, Cristino V, Boaretto R, Argazzi R, Bignozzi CA, Carlo AD (2010) New components for dye-sensitized solar cells. Int J Photoenergy 2010:1–16
Nazeeruddin MK, Péchy P, Renouard T, Zakeeruddin SM, Humphry-Baker R, Comte P et al (2001) Engineering of efficient panchromatic sensitizers for nanocrystalline TiO2-based solar cells. J Am Chem Soc 123:1613–1624
Hagfeldt A, Gratzel M (1995) Light-induced redox reactions in nanocrystalline systems. Chem Rev 95:49–68
Duprez V, Biancardo M, Spanggaard H, Krebs FC (2005) Synthesis of conjugated polymers containing terpyridine-ruthenium complexes: photovoltaic applications. Macromolecules 38:0436–10448
Robel I, Subramanian V, Kuno M, Kamat PV (2006) Quantum dot solar cells. Harvesting light energy with CdSe nanocrystals molecularly linked to mesoscopic TiO2 films. J Am Chem Soc 128:2385–2393
Gratzel M (2003) Dye-sensitized solar cells. J Photochem Photobiol C 4:145–153
Fotsa Ngaffo F, Caricato AP, Fernandez M, Martino M, Romano F (2007) Structural properties of single and multilayer ITO and TiO2 films deposited by reactive pulsed laser ablation deposition technique. Appl Surf Sci 253:6508–6511
Park NG, Vande Lagemaat J, Frank AJ (2000) Comparison of dye-sensitized rutile- and anatase-based TiO2 solar cells. J Phys Chem B 104:8989–8994
Barbe CJ, Arendse F, Comte P, Jirousek M, Lenzmann F, Shklover V, Gratzel M (1997) Nanocrystalline titanium oxide electrodes for photovoltaic applications. J Am Ceram Soc 80:3157–3171
Burnside S, Winkel S, Brooks K, Shklover V, Gratzel M, Hinsch A et al (2000) Deposition and characterization of screen-printed porous multi-layer thick film structures from semiconducting and conducting nanomaterials for use in photovoltaic devices. J Mater Sci Mater Electron 11:355–362
Yang L, Zhang Z, Fang S, Gao X, Obata M (2007) Influence of the preparation conditions of TiO2 electrodes on the performance of solid-state dye-sensitized solar cells with CuI as a hole collector. Sol Energy 81:717–722
Krasˇovec UO, Berginc M, Hocˇevar M, Topic M (2009) Unique TiO2 paste for high efficiency dye-sensitized solar cells. Sol Energy Mater Sol Cells 93:379–381
Huang N, Sebo B, Pan MM, Liu YM, Peng T, Sun WW, Bu CH, Zhao XZ (2013) A viscous titania paste with a single coating-sintering step for 8–24 μm thick, high-haze, high-quality TiO2 films of dye-sensitized solar cells. Sol Energy 97:266–272
Maldonado-Valdivia AI, Galindo EG, Ariza MJ, García-Salinas MJ (2013) Surfactant influence in the performance of titanium dioxide photoelectrodes for dye-sensitized solar cells. Sol Energy 91:263–272
Mohammadi MR, Louca RRM, Fray DJ, Welland ME (2012) Dye-sensitized solar cells based on a single layer deposition of TiO2 from a new formulation paste and their photovoltaic performance. Sol Energy 86:2654–2664
Mohammadi MR, Ghorbani M, Cordero-Cabrera MC, Fray DJ (2006) Synthesis of high surface area nanocrystalline anatase-TiO2 powders derived from particulate sol–gel route by tailoring processing parameters. J Sol-Gel Sci Technol 40:15–23
Kasuga T, Hiramatsu M, Hoson A, Sekino T, Niihara K (1999) Titania nanotubes prepared by chemical processing. Adv Mater 11:1307–1311
Bakhshayesh AM, Mohammadi MR, Dadar H, Fray DJ (2013) Improved efficiency of dye-sensitized solar cells aided by corn-like TiO2 nanowires as the light scattering layer. Electrochim Acta 90:302–308
Ross WD (1971) Theoretical computation of light scattering power: comparison between TiO2 and air bubbles. J Paint Technol 43:50–66
Bakhshayesh AM, Mohammadi MR (2013) The improvement of electron transport rate of TiO2 dye-sensitized solar cells using mixed nanostructures with different phase compositions. Ceram Int 39:7343–7353
Mohammadi MR, Bakhshayesh AM, Sadri F, Masroor M (2013) Improved efficiency of dye-sensitized solar cells by design of a proper double layer photoanode electrodes composed of Cr-doped TiO2 transparent and light scattering layers. J Sol-Gel Sci Technol 67:77–87
Ito S, Liska P, Pechy P, Bach U, Nazeeruddin MK, Kay A, Zekeeruddin SM, Grätzel M (2005) Control of dark current in photoelectrochemical (TiO2/I− I3 −) and dye-sensitized solar cells. Chem Commun 34:4351–4353
Cullity BD (1987) Elements of X-ray diffraction. Addison-Wesley Publishing Company Inc., London
Zhang H, Banfield JF (2000) Understanding polymorphic phase transformation behavior during growth of nanocrystalline aggregates: insights from TiO2. J Phys Chem B 104:3481–3487
Gribb A, Banfield JF (1997) Particle size effects on transformation kinetics and phase stability in nanocrystalline TiO2. Am Mineral 82:717–728
Zhang H, Banfield JFJ (1998) Thermodynamic analysis of phase stability of nanocrystalline titania. Mater Chem 8:2073–2076
Ovenstone J, Yanagisawa K (1999) Effect of hydrothermal treatment of amorphous titania on the phase change from anatase to rutile during calcination. Chem Mater 11:2770–2774
Hu Y, Tsai HL, Huang CL (2003) Effect of brookite phase on the anatase-rutile transition in titania nanoparticles. J Eur Ceram Soc 23:691–696
Gratzel M (2005) Solar energy conversion by dye-sensitized photovoltaic cells. Inorg Chem 44:6841–6851
Wu JM, Shih HC, Wu WT (2006) Formation and photoluminescence of single-crystalline rutile TiO2 nanowires synthesized by thermal evaporation formation and photoluminescence of single-crystalline rutile TiO2 nanowires synthesized by thermal evaporation. Nanotechnology 17:105–109
Shankar K, Basham JI, Allam NK, Varghese OK, Mor GK, Feng X, Paulose M, Seabold JA, Cho KS, Grimes CA (2009) Recent advances in the use of TiO2 nanotube and nanowire arrays for oxidative photoelectrochemistry. J Phys Chem 113:6327–6359
Feigenbrugel V, Loew C, Calvé SL, Mirabel P (2005) Near-UV molar absorptivities of acetone, alachlor, metolachlor, diazinon and dichlorvos in aqueous solution. J Photochem Photobiol A Chem 174:76–81
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moradzaman, M., Mohammadi, M.R. Development of an aqueous TiO2 paste in terms of morphological manipulation of nanostructured photoanode electrode of dye-sensitized solar cells. J Sol-Gel Sci Technol 75, 447–459 (2015). https://doi.org/10.1007/s10971-015-3717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-015-3717-1