Abstract
Inductively coupled plasma mass spectrometry (ICP-MS) has been used for analysis of the bones of archanthropus, ancient bear, southern mammoths, dinosaurs, as well as soils collected in various parts of Uzbekistan. The concentrations of 64 elements have been detected including thorium and uranium for which isotope compositions were also analysed. The comparison of the ICP-MS data with the results we obtained earlier for some bones using instrumental neutron activation analysis (INAA) is presented. The concentrations of 234, 235, 238U are up to two orders of magnitude elevated compared to the soils (e.g.238U in south mammoth bone—130.1 mg/kg, and that of soil is only 1.6 mg/kg). The levels of 236U, and 239, 240, 242, 244Pu isotopes (possible neutron capture products of 235U and 238U) correspond to a count rate of blank samples. In addition, the isotope analysis confirms the uranium in the bones and surrounding soils is natural (the average determined for all samples uranium ratios 235U/238U = 0.0071 ± 0.0003 (2-sigma errors). The concentration of 230Th (which is the part of 234U and 238U decay chain) is also elevated in the bones compared to the surrounding soils, however, the concentrations of stable 232Th largely correspond to those of the soil. The excess uranium detected in bones is most probably due to the preferential accumulation from soil, and not to the paleo diet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The elemental and isotope analysis of prehistoric bones found in large quantities in archeological sites [1,2,3,4,5] is of large practical interest because it can shed light on the prehistoric environment of the species that inhabited that time, as well as help to better understand the present-day processes, such as radionuclides migration and accumulation in different biomaterials, radiation effects including radioactive decay and spontaneous uranium fission. Instrumental neutron activation analysis (INAA) make use of thermal neurons for samples irradiation followed by detection of the gamma transitions of the radioactive isotopes produced, has been previously widely used for elemental analysis of archaeological and environmental samples. Although being a multi-elemental technique not requiring an extensive samples preparation, a very high cost and low sensitivity for some elements e.g. for Pb, S, F, Fe, Nb, and others, has been the main limitations. Over the years several table-top instrumentation techniques have been developed. For instance, Resonance Ionisation Mass Spectrometry (RIMS) that makes use of the tunable lasers for selective ionisation of atoms from the samples and couple the laser ionisation source with the mass spectrometry [6,7,8,9,10] have been widely applied for analysis of e.g. uranium and noble gases in environmental and extraterrestrial samples such as meteorites and chondrules [11,12,13]. Inductively coupled plasma mass spectrometry (ICP-MS), unlike RIMS that is usually tuned to a single- element/ isotope of interest, is a multi-elemental technique having been developed to become commercially available and having almost comparable sensitivity (ppt-ppb). Previous generation apparatuses suffered from a lack of selectivity. However, it has been recently addressed with the development of the tandem ICP-MS/MS employing collision/ reaction cells (He, O2, NH3) for molecular beams manipulation effectively separating isobaric and polyatomic contaminants. Acid digestion is required for analysis of solid (bone) samples. During this process, the samples are crushed into the powders, mixed with a range of concentrated acids, and heated for several hours bringing the sample material into a liquid phase prior to the ICP-MS analysis.
Currently there are few systematic studies of elemental compositions of prehistoric and ancient bone samples with ICP-MS and INAA techniques, presumably due to difficulties in obtaining the samples for destructive analysis which both techniques are. Some examples of INAA elemental analysis of prehistoric bones can be found in our previous works [14, 15].
However, the elemental composition of the present-day bones of various animals is well studied. As early as in 1928 the group of Kramer first quantified the calcium/ phosphorus ratio in various bones [16]. In later works these bones of animals and humans were characterised by a multi-element neutron activation analysis (INAA) [3, 17]. Also, INAA was very useful for detection of many elements on bones, particularly Ca, Na, Mg, Mn, Cl and P concentrations of which were found to correlate with the presence of various bone diseases [14, 15, 18,19,20,21]. The usual radiochemistry technics, e.g. α-spectroscopy, has been also applied for detection of the specific α-activities of environmental radioisotopes of 210Pb, 210Po, and 226Ra in modern bones of animals [19].
In this work we have applied the ICP-MS method and analysed the elemental composition (64 elements) of prehistoric bones and the sample of the soils collected near these bones in different parts of Uzbekistan. We compare these ICP-MS data with our previous INAA measurements of the same samples. We also present the isotope measurements for some actinides (Th, U, and Pu).
Experimental
Prehistoric bone samples analysed
Bone and soil samples were provided by the Archaeological Institute and the Geological Museum, Republic of Uzbekistan. Dinosaur bone was found in Kyzyl-Kum in 2005. Bones of Southern mammoths (Fig. 1) were discovered in 2013 and 2014 in mountainous areas, near the city of Angren and the city of Karshi [1], respectively. Ancient bone remains include finds of a bear and archanthrope (homo sapience) from the ancient site—the cave of Selungur, located on the territory of South Ferghana [1, 2]. Analysed bones and their soils were designated in the following order: MB1 and SMB1, MB2 and SMB2—Southern Mammoth Bones and Soil respectively; DB and SDB—Dinosaurs Bone and Soil; BA—Archanthrope bone; BB—bear bone; STB and SSTB—standard bone and soil (a bone of present time sheep that was placed several meters deep into the local soil and resided there for 10 years before the elemental analysis [3, 17]), respectively. The standard bone was collected in 1966 and resided for 10 years in the same type of soil in Uzbekistan [3, 17]. Surface contamination of the studied bones was cleaned by wiping with a swab moistened with ethanol. Then, fragments of bones and soils were crushed, mixed and sieved through a sieve with a diameter holes 2 mm to obtain a homogeneous powder sample. Presented fragments (or samples) of skeletons by state of preservation have a different state of strength and dilapidation. The natural preservation of osteological materials was evaluated according to a five-point system [4]. The highest score 5 is the natural state of the bone, in which the bone tissue retains sufficient strength, so that its fracture requires tangible efforts. The bones with the lowest score 1 are in such a state in which it crumbles when trying to clean or wash it. And further, the score 4—bone remnants easily broken by directional action, but without directional impact retaining shape and in particular superficial layer, and scores 3, 2 -the bone remains, which usually dry out and crack into smaller fragments after cleaning and washing (Fig. 2).
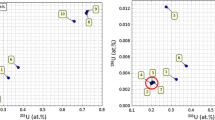
Uranium isotope ratios 235U/238U of various ancient bones and surrounding soils detected with ICP-MS. The natural uranium ratio is indicated with a dashed line. The measurement errors are two sigma errors. MB1 & & SMB1—south mammoth bone found in Angren (2013) and soil collected near this bone respectively; MB2& SMB2—south mammoth bone found in Kashkadari (2014) and soil collected near this bone respectively; BA-arhantrope bone; BB—bone of beer from Selungur cave (1978); DB—Dinosaur bone (2005); SB, STB & SSTB—standard bones (present day sheep bones that was spent 10 years in the same soil) and soil collected near the standard bones
ICP-MS analysis—sample preparation and experimental conditions
For sample preparation, the water purification system is used providing 18.2 MΩ (Type 1 ultrapure laboratory water (Avidity Science)). All acids are purchased of analytical grade and further purified in the laboratory using sub-distillation (DST-1000 acid purification system, Savillex). The purity of the acids and water is regularly checked and confirmed by ICP-MS measurements and < < 1 ppb for most abundant elements. Preparation of the samples for ICP analysis was performed through microwave assisted digestion. The microwave digester was a Mars 5 (CEM) unit, and the 55 mL vessels were Xpress (CEM). The sample procedure followed was: an 100 mg aliquot of the bulk sample was taken into a 55 mL Xpress vessel, 5 mL of sub-distilled nitric acid were added into this aliquot and then heated to 170 °C/1600 W for 45 min in the microwave digester unit. After this step, the digested sample was diluted to 100 mL with water. The solution was subsequently filtered using 0.45 µm, to remove any particles, before being analysed on an ICP-MS/MS system (Agilent 8900 QQQ-ICP-MS). The apparatus is equipped with oxygen gas collision cell which significantly improve detection of elements otherwise suffering from polyatomic and isobaric interferences. The detection was both on atomic (M +) and molecular beams (MO + and MOO +). The data here are reported only for the highest yield ions. The following instrument settings were used: RF power 1550W, nebulizer gas flow 1.09 L/min, spray chamber temperature 2 °C, total rinse time between the samples 30 s (2% nitric and 2% Aqua Regia), flow stabilisation time after rinse 30 s, detection time 10 s with 3 replicates, 100 sweeps per replicate, oxygen cell flow at 24% optimised for the highest uranium oxide ion yields. The concentrations measurements were performed by the comparison to the calibration standards. A linear calibration curves with the range of 0.01–1 ppm were measured before and after the experimental samples. The standard calibration materials were purchased from Sigma-Aldrich (multielement calibration solutions 1–6). Internal standard (Rh, ~ 30 ppb, constant flow) was used for monitoring of any possible signal drift. Separately, the control samples of water with certified concentrations of trace elements were measured together with the calibration standards (SPS-SW1 batch 112, LGC standards). The ICP-MS of the acid digested samples is a well-developed analytical chemistry technique, for which the sensitivity at < ppb with several % precision can be routinely achieved.
We compare ICP-MS results with our data previously obtained using the Instrumental Neutral Activation Analysis (INAA). Typical INAA sample preparation and analytical procedure has been described in detail previously [14, 15]. Briefly, the samples of 300–400 mg were sampled from each bone sample type and were sent for irradiation together with the set of the analytical standards (reference bones with known chemical composition and physical properties from IAEA collection). Prepared samples were placed into aluminium containers and irradiated in nuclear reactor WWR-SM (Institute of Nuclear Physics of the Academy Sciences, Republic of Uzbekistan), with a thermal neutron flux ~ 5 × 1013 cm−2 s−1, exposure varied from 10 s to 15 h, followed by cooling down time from 4 min to 60 days to allow for decay of short-lived nuclides. Concentrations of elements were deduced by comparison to simultaneously irradiated standards, analysing the induced activities using highly pure germanium detector with energy resolution of 1.8 keV at 60Co 1332 keV gamma-transition. The mean squared error of the INAA analysis was estimated as 12–15% [14, 15].
Results and discussion
Elemental composition with ICP-MS and comparison with previous INAA analysis
The Concentrations of the elements in mg/kg in prehistoric bones and soils collected around these bones determined by ICP-MS are presented in the Table 1. The concentrations of 64 elements were determined with high sensitivity. For instance, ICP-MS allowed detection of low concentrations of 9Be—0.1, 101Ru—0.002, 103Rh—0.04, 178Hf—0.04, 185Re—0.003, 193Ir—0.009 и 205Tl—0.002 mg/ kg that is impossible to detect with INAA. Ca and P are the main bone forming elements. From INAA analysis, in prehistoric bones the Ca concentration varies 29.2–41.6%, while the standard bone concentration is 29.2%. The concentrations of 238U were found to be 130.1, 135.8 and 59.8 mg/kg for MB1, MB2 и BB, respectively. At the same time, the concentrations of 232Th in the bones are 0.8, 0.3 and 1.1 mg/ kg for BA, BB и DB, and 6.0, 13.8 and 0.4 mg/kg for MB1, MB2 и STB, respectively.
Many potentially fission isotopes were also detected, the ratios of detected concentrations of prehistoric bones to standard bone PHB min/STB are calculated as Sc -14, As -64, Mo -14, Sr -3, Y -74, Zr -9.1, Ba -29, La -55, Ce -170, Pr -24, Nd -35, Sm -27, Eu -18, Gd -30.
While uranium concentrations in the soils are < 2.2 mg/kg and in the standard bone ≤ 0.2 mg/kg, the uranium concentrations are elevated in the bear (59.8 mg/kg) and archanthrope (19.7 mg/kg) bones. Also, there is a notable elevation in uranium concentrations in mammoth bone (MB1, 130.1 mg/kg) which is higher than in dinosaurs bone DB (19.6 mg/kg) and mammoth bone MB2 (135.8 mg/kg). Though it should be noted that the soil near MB1 has also the highest uranium content of 1.6 mg/kg. The age of these mammoth bones is expected to be at least several tens of millions of years [14, 15]. Unfortunately, the calculations of the ages using e.g. U–Pb or Th–Pb chronometer produce meaningless results, confirming the system bone-soil is not a closed one with the uranium accumulated in the bones from the surrounding soil. This also confirms the hypothesis that the elevated concentrations of uranium and other elements in the ancient bones are due to accumulation processes.
In the Table 2 we compare the results of ICP-MS with our previous measurements using instrumental neutron activation analysis, INAA [14, 15]. This has a practical interest, because the INAA, unlike ICP-MS is more difficult to perform due to complexity of the experiments during which the samples are irradiated with neutrons with the neutron flux that must be estimated. It is also usually difficult and time consuming to repeat the measurements with INAA, unlike in ICP-MS experiments. In the Table 2 the elemental concentrations obtained with INAA and ICP-MS are listed for the bones of mammoth MB2, dinosaur DB, archanthropus BA, standard bone STB and the soil around the standard bone SSTB.
Detection of 31P with INAA is difficult, because when 31P is irradiated by thermal neutrons from a reactor, the short-lived radionuclide 32P is formed with T1/2 = 14.3 days and β max = 1.7 MeV. Phosphorus is also usually a problematic element for ICP-MS because it suffers from the isobaric polyatomic interferences arising in argon plasma (e.g. 14N16O1H+, 15N15N1H+, 15N16O+). It also has only one stable isotope. The detection of bulk elements e.g. calcium and potassium, is more reliable with INAA. Calcium and potassium are easily ionised in plasma, and the detector is usually become saturated at the Ca and K concentrations of just ~ 20 mg/l. One of the practical solutions would be to dilute the samples several orders of magnitude and measure the balk elements separately from the trace elements. In the Table 2 we report the concentrations of bulk elements measured by INAA (Ca, Na, K, Na, Fe). The trace elements, such as Na, K, Sc, Cr, Mn, Co, Zn, As, Sr, La, Sm, Eu, Yb are reported for both INAA and ICP-MS techniques and generally converge within errors. There are some differences in the concentrations of 232Th in the bones of dinosaur, southern mammoth, archanthropus and the standard, with the range of 0.8−0.78 mg/ kg for INAA and 0.4–15.5 mg/ kg for ICP-MS. Despite this, both methods confirm up to two orders of magnitude increase in 238U concentrations in bone remains (e.g. for mammoth bone MB1, from 1.6 mg/kg in soil to 130.1 mg/ kg). Analysis of the distribution of chemical elements in the Earth’s crust [20] and the thorium content in the bones studied in this work, suggests that the main donor of 238U to the bones is the soil, and not the paleo diet (not from nutrition).
Isotope analysis of actinides
The count rates (in counts per second, cps) of 230,232Th, 233, 234, 235, 236, 238U, and 239, 240, 242, 244Pu isotopes detected with ICP-MS are presented in the Table 3. The numbers in brackets are 2-sigma errors. These are compared to the average count rates of 36 blank samples detected within the same experiment. From elemental analysis, 238U content in prehistoric bones was found to be very high. The concentrations of 238U were highest 130 and 135 mg/kg in mammoth bones and bear bone 59.8 mg/kg. This is ~ 2 orders of magnitude higher compared to the surrounding soil containing 0.15–1.6 mg/kg of 238U. The concentrations of stable 232Th in all bones are consistent with those in surrounding soils. However, 230Th which is the product of alpha decay of 234U and, indirectly, of 238U through beta-decaying 234Th and 234Pa, is again elevated compared to the surrounding soils. Some uranium fission isotopes were also detected (La, Ce, Nd, Sm, Eu, Tb, Yb and Lu, As, Br, and Mo). We examine for the potential presence of the uranium neutron capture isotopes. The count rates of 236U, and 239, 240, 242, 244Pu isotopes are at the level of blank samples having the count rate of < 1cps. The uranium in the bones and soils is natural uranium. 235U/238U isotope ratios correspond to the natural values within 2-sigma errors (the average determined for all samples 235U/238U = 0.0071 (0.0003), the uranium isotope ratios of different samples are plotted in the Fig 2).
Conclusions
In this work we have employed ICP-MS and characterised several bones of dinosaur, southern mammoths, ancient bear, and archanthropus, as well as surrounding soils collected in various parts of Uzbekistan. We detect the concentrations of 58 elements and compared the ICP-MS results with the previous measurements of some bones using INAA. A reasonable agreement between the two methods has been observed, with expected better precision of ICP-MS for some trace elements, and INAA as being more suitable for bulk analysis of e.g. Na, Ca and P. In the same ICPMS experiment it was also possible to analyse the isotope compositions of Th and U, and also probe if Pu isotopes were present. The count rate for 239, 240, 242, 244Pu isotopes are < 1 cps correspond to a blank count rate. Similarly, with 236U uranium neutron capture isotope which was not detected. We notice, that unlike 232Th which concentrations largely correlate with those of surrounding soil samples, the concentration of 238U and 230Th (which is a decaying product of 234U and indirectly of 238U) are up to ~ 2 orders of magnitude higher (e.g. for mammoth bone MB2 the concentration of 238U is 135.8 mg/kg vs surrounding soil is 1.1 mg/kg). The amounts of fissile elements (La, Ce, Pr, Nd, Sm, Eu, Yb, Gd and Sc, As, Br, Mo, Sr, Y, Zr) are also much elevated in prehistoric and ancient bones. Thorium content in the bones under study changes insignificantly, which means that the main donor of 238U into the bone is the soil, and not the paleo diet (i.e. not from plants and nutrition). The isotope analysis confirms the uranium in the bones and surrounding soils is natural. The average determined for all samples 235U/238U = 0.0071 ± 0.0003, 2-sigma errors.
Data availability
The authors confirm that all the data supporting the findings of this study are available within this article.
References
KhA Toichiev, KA Krakhmal, UK Abdunazarov (2013) Burials of the south mammoths finding in Uzbekistan. The II Republic Conference in Tashkent 124–127
KA Krakhmal, RA Khalmukhamedova, NN Volozheninov (1996) Paleographic study of the Khaidarkan valley. The origins of the history of the ancient stone age. Tashkent: Fan, 112–121
Bowen HJM (1966) Trace elements in biochemistry. Acad Press, New York, p 241
Antipina Ye (1999) Bone remains of animals from the site of Gorny. Russia Archaeol 1:103–116
Worked bone research group of the international council of zooarchaeology. www.wbrg.net
Strashnov I, Fernando R, Izosimov I (2019) Trace analysis of radioisotopes by laser spectroscopy and mass spectrometry. J Radioanal Nucl Chem 322:1437–1445
Strashnov I, Izosimov I, Gilmour JD, Denecke MA et al (2019) A laser ablation resonance ionisation mass spectrometer (LA-RIMS) for the detection of isotope ratios of uranium at ultra-trace concentrations from solid particles and solutions. J Anal At Spectrom 34(8):1630–1638
Strashnov I, Blagburn DJ, Gilmour JD (2011) A resonance ionization time of flight mass spectrometer with a cryogenic sample concentrator for isotopic analysis of krypton from extraterrestrial samples. J Anal At Spectrom 26(9):1763–1772
Maul J, Berg T, Eberhardt K, Hoog I, Huber G, Karpuk S, Wendt K (2005) A laser desorption/resonance enhanced photoionisation TOF-system for the spatially resolved trace analysis of elements. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 226(4):644–650
Maul J, Strachnov I, Eberhardt K et al (2006) Spatially resolved ultra-trace analysis of elements combining resonance ionization with a MALDI-TOF spectrometer. Anal Bioanal Chem 386:109–118
Strashnov I, Bland PA, Spurný P, Towner MC, Gilmour JD (2013) Times of impacts that deliver samples of Vesta to earth derived from ultrasensitive 81 Kr–Kr cosmic ray exposure age analysis of Eucrites. Geochim Cosmochim Acta 106:71–83. https://doi.org/10.1016/j.gca.2012.11.043
Strashnov I, Gilmour JD (2013) 81Kr-Kr cosmic ray exposure ages of individual chondrules from Allegan. Meteorit Planet Sci 48(12):2430–2440
Strashnov I, Gilmour JD (2014) Resonance ionisation mass spectrometry of krypton and its applications in planetary science. Hyperfine Interact 227(1–3):259–270
Vasidov A, Osinskaya NS, Khatamov Sh, Akhmadshaev A (2008) INAA of prehistoric and ancient bone remains. J Radioanal Nucl Chem 278(2):287–291
Vasidov A, Akhmadshaev A, Osinskaya NS, Saidullaev BJ (2016) Neutron activation and track analysis of the newly found bones of the southern mammoths and dinosaurs. J Radioanal Nucl Chem 310(3):953–958
Kramer B, Shear MJ (1928) Composition of bone. J Biol Chem 79:147–160
Bowen HJM, Gibbons D (1963) Radioactivation analysis. Clarendon Press, Oxford, p 295
Zaichick S, Zaichick V (2012) NAA of Ca, Cl, Mg, Mn, Na and P content in human bone affected by estrogenic sarcoma. J Radioanal Nucl Chem 293(1):241–246
Kadhim YA, Kadhim NF, Ibrahim NK (2019) Determination of alpha rates emitted from animal bones using CN-85 nuclear track detector. Amer J Guan Chem Molec Spectros 3(1):7–11
Kasimov NS, Vlasov DV (2015) Clarkes of chemical elements as comparison standards in ecogeochemistry. Vestnik Moskovskogo Universiteta, seria 5. Geografiya No 2:7–17
Zaichick S, Zaichick V (2010) Human bone as a biological material for environmental monitoring. Int J Environ Health 4(2/3):278–292
Acknowledgements
The authors are grateful to Akhmadjon Akhmadshayev, the Director of the Geological Museum of the Ministry of Geology of the Republic of Uzbekistan, for his support and provision of valuable prehistoric bone artifacts belonging to various periods of evolution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nesmiyan, I., Ragazzon-Smith, A., Saidullaev, B.D. et al. Actinide isotope analysis and trace element composition of bones of prehistoric animals and humans by inductively coupled plasma mass spectrometry (ICP-MS). J Radioanal Nucl Chem (2024). https://doi.org/10.1007/s10967-024-09508-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10967-024-09508-4