Abstract
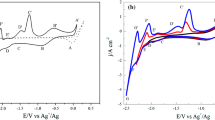
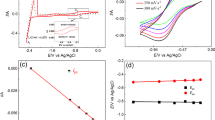
Electroanalytical determination of chlorides in molten salts are often found to not have the accuracy needed for nuclear material monitoring. This was also observed in our studies on samarium trichloride. It was determined that a poorly soluble and relatively stable oxychloride that formed during our studies accounted for the loss of accuracy in the quantification of samarium trichloride. In this study, the spectroscopic and electrochemical properties of synthesized samarium oxychloride were investigated as they relate to the pyrochemical reprocessing of used nuclear fuel in the molten LiCl–KCl of eutectic composition. A qualitative, in situ investigation of oxychloride formation was conducted using electroanalytical voltammetry and Raman spectroscopy. These results were confirmed using synthesized oxychloride. The nature of oxychloride formation, solubility in the molten eutectic, implications in electrochemical processing, and the management of nuclear material in high-temperature systems are discussed.








Similar content being viewed by others
References
Willit JL, Miller WE, Battles JE (1992) Electrorefining of uranium and plutonium–a literature review. J Nucl Mater 195:229–249
Williamson MA, Willit JL (2011) Pyroprocessing flowsheets for recycling used nuclear fuel. Nucl Eng Technol 43:329–334
Cook MT (2015) Hybrid K-edge densitometry as a method for materials accountancy measurements in pyrochemical reprocessing, PhD Dissertation, University of Tennessee Knoxville
Boston CR, Smith GP (1958) Visible and ultraviolet absorption spectra of nickel chloride dissolved in fused LiCl–KCl mixtures. J Phys Chem 62:409–413
Bagri P, Simpson MF (2016) Determination of activity coefficient of lanthanum chloride in molten LiCl-KCl eutectic salt as a function of cesium chloride and lanthanum chloride concentrations using electromotive force measurements. J Nucl Mater 482:248–256
Cho Y-J, Yang H-C, Eun H-C, Kim E-H, Kim I-T (2006) Characteristics of oxidation reaction of rare-earth chlorides for precipitation in LiCl-KCl molten salt by oxygen sparging. J Nucl Sci Technol 43:1280–1286
Zhang C, Rappleye D, Simpson MF (2016) Development and optimization of voltammetry for real time analysis of multi-component electrorefiner salt. ECS Trans 75:95–103
Uda T, Fujii T, Iwadate Y, Uehara A, Yamana H (2013) Raman spectroscopic study of rare earth chlorides in alkali chloride eutectic melts. Z Anorg Allg Chem 639:765–769
Tylka MM, Willit JL, Prakash J, Williamson MA (2015) Method development for quantitative analysis of actinides in molten salts. J Electrochem Soc 162:H625–H633
Bae S-E, Jung TS, Cho Y-H, Kim J-Y, Kwak K, Park T-H (2018) Electrochemical formation of divalent samarium cation and its characteristics in LiCl–KCl Melt. Inorg Chem 57(14):8299–8306
Cordoba G, Caravaca C (2004) An electrochemical study of samarium ions in the molten eutectic LiCl + KCl. J Electroanal Chem 572:145–151
Iizuka M, Inoue T, Shirai O, Iwai T, Arai Y (2001) Application of normal pulse voltammetry to on-line monitoring of actinide concentrations in molten salt electrolyte. J Nucl Mater 297:43–51
Kim TJ, Jung Y, Kim SH, Paek SW, Ahn DH (2011) Elucidation of electrode reaction of EuCl3 in LiCl-KCl eutectic melts through CV curve analysis. Bull Korean Chem Soc 32:863–866
Samin A, Wang Z, Lahti E, Simpson M, Zhang J (2016) Estimation of key physical properties for LaCl3 in molten eutectic LiCl–KCl by fitting cyclic voltammetry data to a BET-based electrode reaction kinetics model. J Nucl Mater 475:149–155
Eun HC, Cho YZ, Son SM, Lee TK, Yang HC, Kim IT, Lee HS (2012) Recycling of LiCl–KCl eutectic based salt wastes containing radioactive rare earth oxychlorides or oxides. J Nucl Mater 420:548–553
Eun HC, Choi JH, Kim NY, Lee TK, Han SY, Jang SA, Kim TJ, Park HS, Ahn DH (2017) A study of separation and solidification of group II nuclides in waste salt delivered from the pyrochemical process of used nuclear fuel. J Nucl Mater 491:149–153
Zygmunt SJ, Mason CFV, Hahn WK (2000) The US plutonium materials conversion program in Russia, International Atomic Energy Agency, https://inis.iaea.org/collection/NCLCollectionStore/_Public/32/033/32033985.pdf
Castrillejo Y, de la Fuente C, Vega M, de la Rosa F, Pardo R, Barrado E (2013) Cathodic behaviour and oxoacidity reactions of samarium (III) in two molten chlorides with different acidity properties: The eutectic LiCl–KCl and the equimolar CaCl2–NaCl melt. Electrochim Acta 97:120–131
Singh V, Chidambaram D (2016) Electrochemical Studies of Lanthanide Chlorides in Molten Eutectic LiCl-KCl, In: Materials Science and Technology 2016, ASM International, Salt Lake City, Utah
Singh V, Chidambaram D (2017) Electrochemical techniques for nuclear safeguards in molten salt. In: 146th Annual Meeting of the Minerals, Metals and Materials Society, ASM International, San Diego, California
Singh V, Chidambaram D (2017) In situ Raman spectroscopy for nuclear material monitoring in molten salt systems, Electrochemical Society Meeting Abstracts MA2017-02, 765–765
Singh VJ, Bruneau CD, Chidambaram D (2019) In situ Raman spectroscopy of samarium ions in molten LiCl-KCl eutectic, ECSarXiv. January 10. https://doi.org/10.1149/osf.io/qpsr3
Sridharan K, Allen T, Anderson M, Simpson M (2012) Thermal Properties of LiCl-KCl Molten Salt for Nuclear Waste Separation, USDOE (United States); Nuclear Energy University Programs (United States), United States of America
Vigier J-F, Laplace A, Renard C, Miguirditchian M, Abraham F (2018) Uranium (III)-Plutonium (III) co-precipitation in molten chloride. J Nucl Mater 499:394–400
Bruneau C, SIngh V, Chidambaram D (2019) Sample preparation for ICP-OES for a more accurate determination of lanthanides in molten salts. Unpublished results
Haeuseler H (1982) Normal coordinate analysis of lanthanoide oxychlorides and oxybromides with Matlockite-type structure. Spectrochim Acta, Part A 38:505–507
Del Cul GD, Nave SE, Begun GM, Peterson JR (1992) Raman spectra of tetragonal lanthanide oxychlorides obtained from polycrystalline and single-crystal samples. J Raman Spectrosc 23:267–272
Acknowledgements
This work was supported by the United States Department of Energy Department of Energy (DOE) under Contracts DE-NE0008236, DE-NE0008572, and DE-NE0008889, and the United States Nuclear Regulatory Commission (NRC) under Contract NRC-HQ-13-G-38-0027. Dr. Kenny Osborne and Ms. Nancy Hebron-Israel serve as the DOE and NRC award program managers, respectively. Materials Characterization Nevada (MCNV) facilities were used for solid-state characterization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singh, V., Bruneau, C., Karmiol, Z. et al. The effect of oxychloride formation on the electroanalytical determination of chlorides in molten salts: an investigation of SmOCl in molten LiCl–KCl. J Radioanal Nucl Chem 332, 691–697 (2023). https://doi.org/10.1007/s10967-023-08800-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08800-z