Abstract
The chemical separation of zirconium from lanthanides by liquid–liquid extraction is challenging but critical for medical and technological applications. Using the example of 89Zr, we optimize the liquid–liquid-extraction process by means of the radiotracer technique. We produced 89Zr by proton irradiation of a metallic yttrium target at a cyclotron. The purification of the radionuclide was performed by a UTEVA resin. 89Zr was separated in no-carrier-added form in a sulfuric acid solution. 89Zr was successfully used in solvent extraction tests with calixarenes for the separation of zirconium from lanthanides. This reaction is suitable for the efficient extraction and purification of lanthanides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The zirconium-89 radionuclide is widely used radiotracer in different fields of interest. Medical applications include immuno imaging, cell imaging, tumor cell therapy, antibody imaging and pharmacokinetics [1,2,3,4,5,6]. In geochemical and environmental studies zirconium-89 is used as a surrogate for tetravalent actinides e.g. in the field of aqueous chemistry, surface chemistry, migration behavior in nuclear waste repositories, thermodynamics, kinetics [7,8,9,10].
The motivation of the present work is the development of methods for the extraction and purification of lanthanides within the ore processing. The processing and separation of the ore leachates is a challenging and expensive process due to the similarity of the chemical and physical properties of the lanthanides and the contaminants. This study focuses on the utilization of calixarenes as highly selective and cost effective extracting agent for the separation of zirconium and lanthanides from ore leachates by means of liquid/liquid extraction. Calixarenes are cyclic organic macromolecules, have a cage structure and can act as a host for guest ions, depending on pH and the ion size [11,12,13,14,15]. Zr is a likely major contaminant of the lanthanides and also acts as surrogate for the ore-associated tetravalent actinides [16, 17]. The use of the radiotracer zirconium-89 allows for the easy access to the highly complex system. Without extensive sample preparation a detailed quantification of Zr and kinetic studies become possible and allow the optimization and parameterization of the chemical-technological process.
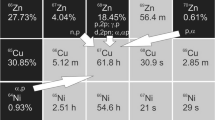
89Zr decays by positron emission (T1/2 = 78.41 h, Iβ+ = 22.74%, Eβ+,end-point = 902 keV) and by electron capture (77.26%, Iγ = 99.04%, Eγ = 909.15 keV) to stable 89Y [18]. Several nuclear reactions, mainly in order to produce 89Zr as a theragnostic radionuclide have been published. Possible nuclear reactions are discussed: 89Y(p,n)89Zr, 89Y(d,2n)89Zr, natZr(p,pxn)89Zr, natSr(α,xn)89Zr and 90Zr(n,xn)89Zr [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33]. In the present study, 89Zr was produced at an in-house cyclotron via the nuclear reaction 89Y(p,n)89Zr. By utilizing ion exchange chromatography techniques, we investigate purification processes using UTEVA resin material [34]. Specifically, we analyze the purification efficiency.
Experimental
Material and methods
The yttrium foil with natural isotope composition (100% 89Y) was purchased from Goodfellow (Germany). The target aluminium plate (AlMg3, Stahl Center Leipzig, Germany) and the aluminium foil (Goodfellow, Germany) had a purity of 96.2 and 99.999%, respectively. The UTEVA Resin was purchased from TrisKem International (France) with a grain size of 50 µm–100 µm [35]. The calixarenes 5,11,17,23-Tetrakis-p-tert-butyl-25,27-bis[3-diethoxyphosphoryl)-propoxy]-26,28-bis(carboxymethoxy)-calix[4]arene (L1) and 5,11,17,23-Tetra-p-tert-butyl-25,27-bis[(8-hydroxyquinoline-carbaldehyde-hydrazone-carbonylmethoxy)]-26,28-dihydroxy-calix[4]arene (L2) were synthesized and characterized (NMR, IR, UV/Vis and ESI–MS) at the Institut für Anorganische Chemie (Universität Leipzig, Germany), respectively (see Fig. 1) [36, 37]. These calixarenes were chosen due to the prediction of their high complex stability constants.
All other chemicals used were obtained from Sigma-Aldrich, Merck and Fluka. All solutions were prepared with deionized water of a resistivity not less than 18.2 MΩ/cm. An ICP-MS (inductively coupled plasma mass spectrometer, Element XR, Thermo Scientific, UK) was used in preliminary tests with the non-radioactive material. γ-ray spectroscopy was performed with an ORTEC detector (dspec pro, GEM-40190P, GammaVision A66-B32, Germany). The detector was calibrated with a 152Eu standard (PTB 443–88, PTB, Germany) in the energy range from 50 to 1500 keV. The fractions of the solvent extraction were measured with a γ-counter (Wizard 3′′, PerkinElmer, Germany) at 909.15 keV, which was also calibrated with the 152Eu standard.
Irradiation
The Y target was prepared by inserting an yttrium foil (12.5 mm × 12.5 mm, 150 µm thick) in a cavity of an aluminium plate with a diameter of 13 mm and a depth of 150 µm. The foil was mechanically fixed by imposing a 2 mm thick aluminium frame. The proton irradiation was performed at our in-house cyclotron “Cyclone 18/9®” (IBA Molecular, Belgium) with a Nirta®Solid target irradiation station (IBA Molecular, Belgium). The maximal current was 22 µA (mean 19 µA) at 18 MeV proton energy on the complete target system. A 12.5 µm titanium window was used to separate the high vacuum system from the helium cooling system. Additionally, the target was cooled with water on its backside (13 °C). The yttrium foil was covered with a 900 µm aluminium foil, where the nuclear reactions 89Y(p,2n)88Zr and 89Y(p,pn)88Y should not be in consideration (Ep < 11.7 MeV) due to their high threshold energy (Table 1) [25, 28]. The exit proton energy was calculated as ~ 10.1 MeV at the end of the target material. The irradiation time was 70 min. As a byproduct, 89mZr (T1/2 = 4.16 min) was produced by the nuclear reaction 89Y(p,n)89mZr (Table 1) [18, 21]. After decay of the short-lived radionuclide 89mZr for 60 min the irradiated yttrium foil was quantitatively dissolved in 2 mL concentrated HNO3. This solution was evaporated to dryness.
Chemical separation of 89Zr
The dried Y(NO3)3 with 89Zr was dissolved in 2 mL 9 M HNO3 (Fig. 2). The UTEVA resin was triple preconditioned with 2 mL 9 M HNO3. The prepacked column had a length of 26 mm and a diameter of 10 mm and was filled with 0.80 g (density 0.39 g/mL) of the resin. The 2 mL Y/89Zr solution was passed through the column by using a syringe at a flow of ~ 0.6 mL/min. The adsorbed 89Zr was washed five times with 2 mL 9 M HNO3 (fractions 1–6). To optimize the Y/Zr separation, preparatory experiments with non-radioactive Y/Zr mixtures were measured by ICP-MS. Finally, the 89Zr was eluted from the resin with 6 bed column volumes of 0.1 M oxalic acid (fractions 7–12). All fractions (2 mL) were measured using γ-ray spectroscopy for determining the 89Zr (909.15 keV) content. The combined Zr-solutions were evaporated to dryness, fumed with a few drops of concentrated H2SO4 to get rid of the oxalic acid, and the 89Zr was dissolved four times with 250 µL 1 M H2SO4 (1 mL in sum). The radionuclide purity was determined by γ-ray spectroscopy and the quantification of the amount of 89Zr in this solution was achieved by measuring the 909.15 keV-peak. The detection limit was 30 Bq/mL at the energy of 909.15 keV.
Solvent extraction with calixarenes
The calixarenes L1 or L2 were dissolved in chloroform to give a solution of 100 µM. The aqueous solution were prepared by adding ~ 5 kBq/mL 89Zr to a non-radioactive Zr-solution (10 µM ZrOCl2.8H2O) at the desired pH (1–5) in diluted H2SO4. 3 mL of the aqueous solution was shaken with 3 mL of the organic phase in a stoppered vial on a horizontal shaker at 300 min−1. The phases were allowed to equilibrate for 60 min. After separation of the phases for 1 min, an aliquot of 2 mL of the aqueous phase were measured by γ-counting in comparison to 2 mL of the aqueous solution before extraction. The percentage extraction %E was calculated by means of the two activities (Ainitial = 89Zr in aqueous solution before extraction, Afinal = 89Zr in aqueous solution after extraction) by using the Eq. (1)
The distribution coefficient D can be calculated by Eq. (2).
Results and discussion
Preparation of 89Zr: The separation efficiency of 89Zr from the Y target by chromatography is shown in Fig. 3. Y is washed from the resin within the first 5 fractions (1–6 in Fig. 3) with 9 M HNO3. 89Zr and the non-radioactive Zr were eluted within the first oxalic acid fraction (7–12 in Fig. 3). No 89Zr could be found in the HNO3 fractions with a detection limit of 30 Bq/ml. Fraction 7 was evaporated and the residue was fumed to dryness with sulfuric acid. The 89Zr was dissolved in dilute sulfuric acid and was used for the solvent extraction tests. The radioactivity was measured and calculated to be 414 MBq ± 20 MBq (25.7 MBq/µAh) at the end of the irradiation. The dissolution of the irradiated target, the separation of 89Zr and the fuming with sulfuric acid are performed within ~ 6 h.
Chromatographic separation of irradiated Y and 89Zr with UTEVA resin; elution of Y and washing of the adsorbed 89Zr with 9 M HNO3 (fractions 1–6); elution of 89Zr with 0.1 M oxalic acid (fractions 7–12); 2 mL each fraction; flux 0.6 mL/min; comparison of a non-radioactive solution (80 mg/L Y; 1 mg/L Zr) measured by ICP-MS and the radiochromatogram with 414 MBq 89Zr
Application of 89Zr: After adding the radiotracer to a non-radioactive Zr solution, the solvent extraction behaviour with L1 and L2 was tested between pH 0.15 and pH 4. Figure 4 illustrates the strong pH dependence on Zr extraction independently of which calixarene is used. The extraction at pH 4 is between 90–95% for both calixarenes. In comparison to an experiment with Eu3+ [36], it is possible to separate Zr from the lanthanides in the region of pH ~ 3.5 (Fig. 4). The pH1/2 values (50% extraction of the elements) are 2.5 and 5.0 for Zr and Eu, respectively. Consequently, we established a protocol for the purification of the lanthanides from a Zr contamination, involving adjustment of the pH first to pH ~ 3.5, followed by reextraction of Zr at pH ~ 0.25. Afterwards the lanthanides are be extracted with the identical calixarene solution at higher pH values (6–7) from the other contaminants in the ore leachates, e.g. iron and aluminium.
Solvent extraction of [89Zr]Zr with the calixarenes L1(circles) and L2(squares) in dependence on pH. Comparison with [152Eu]Eu3+ and L2(triangles) [36]
Summary and conclusions
A fast and quantitative method for the production and purification of 89Zr was implemented at our research site, and enables a prompt availability of 89Zr. Within a single day it is possible to irradiate a few mg-scale Y foils and separate the radionuclide 89Zr with UTEVA resin in a no-carrier-added solution in the range of a few hundred MBq/mL concentration range with a radiochemical yield of 100% ± 8% (n = 3) and a radionuclide purity > 99%. For investigation in solvent extraction with calixarenes, 89Zr was used as radiotracer to optimize the separation from lanthanides. The fast availability of 89Zr provides the opportunity for studying multiple natural and technical systems, including geochemical behavior and ore processing, the mobility of Zr-nanoparticles and medical application methods.
References
Das SS et al (2017) Production and radiochemical separation of a potential immuno-PET imaging agent 89Zr from proton irradiated natY target. J Radioanal Nucl Chem 313:641–645. https://doi.org/10.1007/s10967-017-5316-9
Sato N et al (2015) 89Zr Oxine Complex PET Cell Imaging in Monitoring Cell-based Therapie. Radiology 275:490–500. https://doi.org/10.1148/radiol.15142849
Walther M et al (2011) Implementation of 89Zr production and in vivo imaging of B-cells in mice with 89Zr-labeled anti-B-cell antibodies by small animal PET/CT. Appl Radiat iost 69(6):852–857
Deri MA et al (2013) PET imaging with 89Zr: From radiochemistry to the clinic. Nucl Med Biol 40:3–14. https://doi.org/10.1016/j.nucmedbio.2012.08.004
Verel I et al (2003) 89Zr Immuno-PET: Comprehensive Procedures for the Production of 89Zr-Labeled Monoclonal Antibodies. J Nucl Med 44:1271–1281
Kasbollah A et al (2013) Review on Production of 89Zr in a Medical Cyclotron for PET Radiopharmaceuticals. J Nucl Med Technol 41:35–41. https://doi.org/10.2967/jnmt.112.111377
Altmaier M et al (2013) Recent Advances in Aqueous Actinide Chemistry and Thermodynamics. Chem Rev 113:901–943. https://doi.org/10.1021/cr300379w
Holliday KS et al (2011) Environmental behavior of ZrO2-MgO ceramics containing UO2. Radiochim Acta 99:799–806. https://doi.org/10.1524/ract.2011.1884
Pin C, Joannon S (2002) Combined cation-exchange and extraction chromatography for the concomitant separation of Zr, Hf, Th, and the Lanthanides from geological materials. Talanta 57:393–403. https://doi.org/10.1016/S0039-9140(02)00040-1
Raslan MF, El-Feky MG (2012) Radioactivity and mineralogy of the altered granites of the Wadi Ghadir shear zone, South Eastern Desert. Egypt Chin J Geochem 31:30–40. https://doi.org/10.1007/s11631-012-0546-6
Mohhtari B et al (2011) A review of calixarene applications in nuclear industries. J Radioanal Nucl Chem 287:921–934. https://doi.org/10.1007/s10967-010-0881-1
Arnaud-Neu F et al (1999) Extraction and Complexation of Alkali, Alkaline Earth, and f-Element Cations by Calixaryl Phosphine Oxides. Chem Eur J 5:175–186. https://doi.org/10.1002/(SICI)1521-3765(19990104)5:1%3c175::AID-CHEM175%3e3.0.CO;2-P
Bauer A et al (2019) Multidentate extracting agents based on calix[4]arene scaffold – UVI/EuIII separation studies. Sep Purif Technol 213:246–254. https://doi.org/10.1016/j.seppur.2018.12.041
Huang H et al (2014) Extraction of trivalent americium and europium from nitric acid solution with a calixarene-based diglycolamid. Sep Purif Technol 123:235–240. https://doi.org/10.1016/j.seppur.2013.12.039
Lu X et al (2017) Reactive extraction of europium(III) and neodymium(III) by carboxylic acid modified calixarene derivatives: Equilibrium, thermodynamics and kinetics. Sep Purif Technol 188:250–259. https://doi.org/10.1016/j.seppur.2017.07.040
Voncken, J. H. L., 2016. The Rare Earth Elements, first ed., SpringerBriefs in Earth Sciences, ebook. https://www.springer.com/de/book/9783319268071
Eichholz GG et al (1953) The determination of uranium and thorium in ores. Can J Phys 31:613–628. https://doi.org/10.1139/p53-058
NuDat 2.7 database. 2015. https://www.nndc.bnl.gov/nudat2/ (accessed 22 April 2020).
Sadeghi M et al (2012) Accelerator production of the positron emitter zirconium-89. Ann Nucl Energy 41:97–103. https://doi.org/10.1016/j.anucene.2011.11.014
Kandil SA et al (2007) A comparative study on the separation of radiozirconium via ion-exchange and solvent extraction techniques, with particular reference to the production of 88Zr and 89Zr in proton induced reactions on yttrium. J Radioanal Nucl Chem 274:45–52. https://doi.org/10.1007/s10967-006-6892-2
Uddin MS et al (2005) Experimental studies on excitation functions of the proton-induced activation reactions on yttrium. Appl Radiat Isot 63:367–374. https://doi.org/10.1016/j.apradiso.2005.04.006
Yang S et al (2017) Production cross sections of proton-induced reactions on yttrium. Nucl Instr Meth B 398:1–8. https://doi.org/10.1016/j.nimb.2017.03.021
Sharifian M et al (2017) Modeling and experimental data of zirconium-89 production yield. Appl Radiat Isot 130:206–210. https://doi.org/10.1016/j.apradiso.2017.09.044
Omara HM et al (2009) Proton induced reactions on 89Y with particular reference to the production of the medically interesting radionuclide 89Zr. Radiochim Acta 97:467–471. https://doi.org/10.1524/ract.2009.1645
Khandaker MU et al (2012) Investigations of 89Y(p, x)86,88,89gZr, 86m+g,87g,87m,88gY, 85gSr, and 84gRb nuclear processes up to 42 MeV. Nucl Instr Meth B 271:72–81. https://doi.org/10.1016/j.nimb.2011.11.009
Steyn GF et al (2011) Excitation Functions of Proton Induced Reactions on 89Y and 93Nb with Emphasis on the Production of Selected Radio-Zirconiums. J Korean Phys Soc 59:1991–1994. https://doi.org/10.3938/jkps.59.1991
Mustafa MG et al (1988) Measurements and a direct-reaction–plus–Hauser-Feshbach analysis of 89Y(p, n)89Zr, 89Y(p,2n)88Zr, and 89Y(p, pn)88Y reactions up to 40 MeV. Phys Rev C 38:1624–1637. https://doi.org/10.1103/PhysRevC.38.1624
Zweit J et al (1991) Production of no-carrier-added zirconium-89 for positron emission tomography. Appl Radiat Isot 42:199–201. https://doi.org/10.1016/0883-2889(91)90074-B
Tang Y et al (2016) A simple and convenient method for production of 89Zr with high purity. Appl Radiat Isot 118:326–330. https://doi.org/10.1016/j.apradiso.2016.09.024
Uddin MS et al (2008) Excitation functions of the proton induced nuclear reactions on natural zirconium. Nucl Instr Meth B 266:13–20. https://doi.org/10.1016/j.nimb.2007.10.010
Kandil SA et al (2007) Excitation functions of (α, xn) reactions on natRb and natSr from threshold up to 26 MeV: Possibility of production of 87Y, 88Y and 89Zr. Appl Radiat Isot 65:561–568. https://doi.org/10.1016/j.apradiso.2006.12.007
Ivanov PI et al (2014) Cyclotron production and radiochemical purification of 88,89Zr via α-particle induced reactions on natural strontium. Appl Radiat Isot 90:261–264. https://doi.org/10.1016/j.apradiso.2014.04.018
Lewis VE, Zieba KJ (1980) A transfer standard for d + t neutron fluence and energy. Nucl Instr Meth 174:141–144. https://doi.org/10.1016/0029-554X(80)90422-X
Kazakov AG et al (2018) Separation of 89Zr from irradiated yttrium targets by extraction chromatography. J Radioanal Nucl Chem 317:605–611. https://doi.org/10.1007/s10967-018-5888-z
Horwitz EP et al (1992) Separation and preconcentration of uranium from acidic media by extraction chromatography. Anal Chim Acta 266:25–37. https://doi.org/10.1016/0003-2670(92)85276-C
Jäschke A et al (2017) Hydroxyquinoline-Calix[4]arene-Conjugates as Ligands for Polynuclear Lanthanide Complexes: Preparation, Characterization, and Properties of a Dinuclear Eu(III) complex. Eur J Inorg Chem 2017:894–901. https://doi.org/10.1002/ejic.201601326
Kersting B, Lehmann U (2009) Chemistry of Metalated Container Molecules. Adv Inorg Chem 61:407–470. https://doi.org/10.1016/S0898-8838(09)00207-4
Acknowledgements
We sincerely thank the Bundesministerium für Bildung und Forschung (project SE-FLECX, grant #033R132A) for funding of this work. We are thankful to Claudia Schößler (HZDR) for the ICP-MS measurements, Stefan Gruhne (HZDR) for the mechanical manufacturing of the aluminium target system and Astrid Jäschke and Florian Glasneck (University Leipzig) for the provision of the calixarenes.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mansel, A., Franke, K. Production of no-carrier-added 89Zr at an 18 MeV cyclotron, its purification and use in investigations in solvent extraction. J Radioanal Nucl Chem 328, 419–423 (2021). https://doi.org/10.1007/s10967-021-07634-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-021-07634-x