Abstract
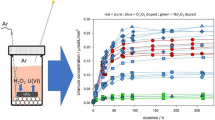
The mechanism of dissolution of sintered UO2 fuel pellets in nitric acid is the essential starting input information required for the design of a continuous dissolution system for spent nuclear fuel reprocessing. The current article establishes the mechanism of this reaction under typical PUREX process conditions based on the concentration profiles of nitric & nitrous acids and NO X gas composition during the course of dissolution of sintered UO2 pellets in nitric acid. The results would be useful in optimizing the dissolution process and improving the throughput of the dissolution system.














Similar content being viewed by others
References
Department of Atomic Energy Annual Report 2011–2012 (http://dae.nic.in/writereaddata/ar2012_0.pdf)
Uriarte AL, Rainey RH (1965) Dissolution of high-density UO2, PuO2, and UO2-PuO2 pellets in inorganic acids; technical report ORNL-3695. Union Carbide Corporation, Oak Ridge National Laboratory, Houston
Shying ME, Florence TM, Carswell DJ (1970) Oxide dissolution mechanisms—I: the role of fluoride in the thoria/nitric/hydrofluoric acid system. J Inorg Nucl Chem 32:3493–3508
Shying ME, Florence TM, Carswell DJ (1972) Oxide dissolution mechanisms—II: a mechanism for the thoria/nitric/hydrofluoric acid system. J lnorg Nucl Chem 34:213
Schiefelbein GF, Lerch RE (1971) “Dissolution properties of UO2-PuO2 thermal reactor fuels”, PNNL report BNWL-1581
Ryan JL, Bray LA (1980) “Dissolution of plutonium dioxide—a critical review”. In: JD Navratil, WW Schultz (eds.) Actinide separations. ACS Symposium Series, 17th ACS National Meeting, Honolulu, HI, 1980,117, Chapter 35, pp 499–514
De Regge P, Huys D, Ketels J, Vandevelde L, Baetsle LH (1979) Dissolution of mechanically mixed MOX fuel and insoluble residue characteristics, FRFR proceedings of a symposium held at UKAEA, pp. 133–143
Berger P (1990) Study of the mechanism of the dissolution of actinide dioxides (UO2, NpO2, PuO2, AmO2) by chemical or electrochemical redox reactions in aqueous acid medium, RAPPORT CEA-R-5515
Serrano J, Glatz J, Toscano E, Papaioannou D, Barrero J, Coquerelle M (1998) Influence of low temperature air oxidation on the dissolution behaviour of UO2 and MOX spent fuel. J Alloys Comp 271–273:573–576
Taylor RF, Sharratt EW, Chazal LE, Logsdail DH (1963) Dissolution rate of uranium dioxide sintered pellets in nitric acid systems. J Appl Chem 13:32–40
Shabbir M, Robbins RG (1968) Kinetics of the dissolution of uranium dioxide in nitric acid. I. J Appl Chem 18:129–134
Shabbir M, Robbins RG (1969) Kinetics of the dissolution of uranium dioxide in nitric acid. II. J Appl Chem 41:126–134
Inoue A, Tsujino T (1984) Dissolution rates of U3O8 powders in nitric acid. Ind Eng Chem Process Des Dev 23:122–125
Wen CY (1968) Noncatalytic heterogeneous solid fluid reaction models. Ind Eng Chem 60:34–54
Inoue Akihiko (1986) Mechanism of the oxidative dissolution of UO2 in HNO3 solution. J Nucl Mat 138:152–154
Fukasawa T, Ozawa Y (1986) Relationship between dissolution rate of uranium dioxide pellets in nitric acid solutions and their porosity. J Radioanal Nucl Chem Lett 106(6):345–356
Fukasawa T, Ozawa Y, Kawamura F (1991) Generation and decomposition behaviour of HNO2 during UO2 dissolution. Nuc Tech 94:108–113
Takashima Y, Kumagai M, Matsumoto S, Wakabayashi M, Fukudome K (1987) RECOD 87(2):569
Nishimura Kenji, Chikazawa Takahiro, Hasegawa Shinichi, Tanaka Hiroshi, Ikeda Yasuhisa, Yasuike Yoshiyuki, Takashima Yoichi (1995) Effect of nitrous acid on dissolution of UO2 powders in nitric acid. J Nucl Sci Tech 32(2):157–159
Ikeda Y, Yasuike Y, Nishimura K, Hasegawa S, Takashima Y (1995) Kinetic study on dissolution of UO2 powders in nitric acid. J Nucl Mat 224:266–272
N. Desigan, N.K. Pandey, U. Kamachi Mudali, J.B. Joshi, “Role of nitrous acid during the dissolution of UO2 in nitric acid”, SESTEC 2016 Proceedings, page 198, SESTEC 2016 held at IIT Guwahati from May 17–20, 2016
Ikeda Yasuhisa, Yasuike Yoshiyuki, Takashima Yoichi, Nishimura Kenji, Hasegawa Shinichi (1993) Acceleration effect of noble metals on dissolution rate of UO2 powders in nitric acid. J Nucl Sci Tech 30(5):485–487
Homma Shunji, Koga Jiro, Matsumoto Shiro, Kawata Tomio (1993) Dissolution rate equation of UO2 pellet. J Nucl Sci Tech 30(9):959–961
Ikeda Yasuhisa, Yasuike Yoshiyuki, Takashima Yoichi, Park YY, Asano Y, Tomiyasu H (1993) 17O NMR study on dissolution reaction of UO2 in nitric acid—mechanism of electron transfer. J Nucl Sci Tech 30(9):962–964
Steward SA, Gray WJ (1994) “Comparison of uranium dissolution rates from spent fuel and uranium dioxide” LLNL report UCRL-JC-115555
Steward SA, Homer C (1993) Weed, “Modeling of UO2 aqueous dissolution over a wide range of conditions”. MRS Proc 333:409. doi:10.1557/PROC-333-409
Steward SA, Mones ET (1994) “Aqueous dissolution rates of uranium oxides”, M95005920—UCRL-JC-119118
Steward SA, Mones ET (1996) Comparison and modelling of aqueous dissolution rates of various uranium oxides—M97051537—UCRL-JC-124602
Pierce EM, Icenhower JP, Serne RJ, Catalano JG (2005) Experimental determination of UO2(cr) dissolution kinetics—effects of solution saturation state and pH”. J Nucl Mat 345:206–218
Amme M, Svedkauskaite J, Bors W, Murray M, Merino J (2007) A kinetic study of UO2 dissolution and H2O2 stability in the presence of groundwater ions. Radiochim Acta 95:683–692. doi:10.1524/ract.2007.95.12.683
Desigan N et al (2015) Dissolution kinetics of Indian PHWR UO2 fuel pellets in nitric acid—effect of initial acidity and temperature. Prog Nucl Ener 83:52–58
Florence TM, Farrar Y (1963) Spectrophotometric determination of uranium with 4-(2-Pyridylazo) resorcinol. Anal Chem 35(11):1613–1616
Desigan N et al (2015) “Development of an analytical method for estimating the composition of NOX gas using ion chromatography”, poster presentation in NUCAR 2015 National Symposium held at Mumbai from 09–13 Feb 2015
Sakurai Tsutomu et al (1998) The composition of the NOX generated in the dissolution of uranium dioxide. Nucl Tech 83:24–30
Kobayashi H et al (1976) The mechanism of nitrous acid decomposition. Nippon Kagaku Kaishi 3:383. doi:10.1246/nikkashi.1976.383
Doraiswami LK, Sharma MM (1984) Heterogeneous reactions: analysis, examples and reactor design, vol II. Wiley, New York
Park JY, Lee YN (1988) Solubility and decomposition kinetics of nitrous acid in aqueous solution. J Phys Chem 92(22):6294–6302. doi:10.1021/j100333a025
Parkt Jong-Yoon, Lee Yin-Nan (1988) Solubility and decomposition kinetics of nitrous acid in aqueous solution. J Phys Chem 92:6294–6302
Karraker David G (1985) Oxidation of hydrazine by nitric acid. Inorg Chem 24:4470–4477
Zacharia IG, Deen WM (2005) Diffusivity and solubility of nitric oxide in water and saline. Ann Biomed Eng 33:214–222. doi:10.1007/s10439-005-8980-9
Acknowledgements
This work forms a part of the PhD thesis of Desigan who acknowledges HNBI for giving him an opportunity to pursue his research work. He also acknowledges the support given by the management of IGCAR in carrying out the experimental work pertaining to this research project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Desigan, N., Bhatt, N.P., Pandey, N.K. et al. Mechanism of dissolution of nuclear fuel in nitric acid relevant to nuclear fuel reprocessing. J Radioanal Nucl Chem 312, 141–149 (2017). https://doi.org/10.1007/s10967-017-5208-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5208-z