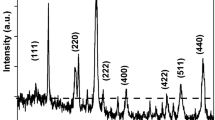
Abstract
The effect of coating with nine different carboxylic acids (glycolic, propionic, lactic, malic, tartaric, citric, mandelic, caproic and caprylic) on nanostructured magnetite (D ~ 10 nm) was studied by Raman and photoacoustic, magnetic and 57Fe Mössbauer measurements. Mössbauer spectra of frozen suspensions showed dominantly magnetically split envelopes at lower temperatures, which were evaluated by hyperfine field distribution method. Mössbauer and Raman spectroscopy indicated similar variation of relative occurrence of magnetite and maghemite phases. These results are discussed on the basis of the hypothesis that different carboxylic acids can promote either the oxidation or reduction of iron oxide nanoparticles.









Similar content being viewed by others
References
Radad K, Al-Shraim M, Moldzio R, Rausch WD (2012) Environ Toxicol Pharmacol 34(3):661–672
Chen CL, Zhang H, Ye Q, Hsieh HY, Hitchens TK, Shen HH, Liu L, Wu YJ, Foley LM, Wang SJ, Ho C (2011) Mol Imaging Biol 5:825–839
Lunacek J, Lesnak M, Jandacka P, Dvorsky R, Repkova J, Seidlerova J, Vitkovska N (2015) Sep Sci Technol 50(16):2606–2615
Holban AM, Grumezescu AM, Gestal MC, Mogoanta L, Mogosanu GD (2013) Curr Org Chem 18(2):185–191
Chandra S, Mehta S, Nigam S, Bahadur D (2010) New J Chem 34:648–655
Sui YC, Skomski R, Sorge KD, Sellmyer DJ (2004) J Appl Phys 95:7151
Lakshmanan R, Okoli C, Boutonnet M, Järås S, Rajarao GK (2013) Bioresour Technol 129:612–615
Estelrich J, Escribano E, Queralt J, Busquets MA (2015) Int J Mol Sci 16:8070–8101
Voit W, Kim DK, Zapka W, Muhammed M, Rao KV (2001) MRS Proceedings (2001), vol 676. Cambridge University Press, Cambridge
Albrecht T, Bührer C, Fähnle M, Maier K, Platzek D, Reske J (1997) Appl Phys A 65(2):21
Yildirimer L, Thanh NTK, Loizidou M, Seifalian AM (2011) Nano Today 6(6):585–607
Soenen SJ, De Cuyper M, De Smedt SC, Braeckmans K (2012) Methods Enzymol 509:195–224
Lei L, Ling-Ling J, Yun Z, Gang L (2013) Chin Phys B 22(12):127503
Wu W, Wu Z, Yu T, Jiang C, Kim WS (2015) Sci Technol Adv Mater 16(023501):43
Khalil IM (2015) Arab J Chem 8(2):279–284
Ray S, Nath SK, Kumar A, Agarwala RC, Agarwala V, Chaudhari GP, Daniel BSS (2009) Adv Mater Res 67:221–226
Chen D, Ni S, Chen Z (2007) China Particuol 5(5):357–358
Sasaki T, Zeng X, Koshizaki N (1998) MRS Proceedings (1998), vol 526, Cambridge University Press, Cambridge
Beketov IV, Safronov AP, Medvedev AI, Alonso J, Kurlyandskaya GV, Bhagat SM (2012) AIP Adv 2:022154
Obayemi JD, Dozie-Nwachukwu S, Danyuo Y, Odusanya OS, Anuku N, Malatesta K, Soboyejo WO (2015) Mater Sci Eng, C 46(1):482–496
Elblbesy MAA, Madbouly AK, Hamdan TAA (2014) Am J Nano Res Appl 2(5):98–103
Khalafalla E, Reimers G (1980) IEEE Trans Magn 16:178
Regmi R, Black C, Sudakar C, Keyes PH, Naik R, Lawes G, Vaishnava P, Rablau C, Kahn D, Lavoie M, Garg VK, Oliveira AC (2009) J Appl Phys 106:113902
Racuciu M, Creanga DE, Airinei A, Badescu V, Apetroaie N (2007) Magnetohydrodynamics 43(4):11–18
de Sousa ME, Fernández van Raap MB, Rivas PC, Zélis PM, Girardin P, Pasquevich GA, Alessandrini JL, Muraca D, Sánchez FH (2013) J Phys Chem C 117:5436–5445
Soler MAG, Alcantara GB, Soares FQ, Viali WR, Sartoratto PPC, Fernandez JRL, da Silva SW, Garg VK, Oliveira AC, Morais PC (2007) Surf Sci 601(18):3921–3925
Goloverda G, Jackson B, Kidd C, Kolesnichenko V (2009) J Magn Magn Mater 321(10):1372–1376
Wei X, Wei Z, Zhang L, Liu Y, He D (2011) J Colloid Interface Sci 354:76–81
Burdukova E, Ishida N, Shaddick T, Franks GV (2011) J Colloid Interface Sci 354:82–88
Szekeres M, Tóth IY, Illés E, Hajdú A, Zupkó I, Farkas K, Oszlánczi G, Tiszlavicz L, Tombácz E (2013) Int J Mol Sci 14:14550–14574
Kazmierczaka M, Pogorzelec-Glasera K, Hilczera A, Jurgab S, Majchrzyckic Ł, Nowickic M, Czajkac R, Matelskic F, Pankiewiczd R, Łeskad B, Kepinskie L, Andrzejewskia B (2014) Mater Technol 48:59–62
Daou TJ, Pourroy G, Begin-Colin S, Greneche JM, Ulhaq-Bouillet C, Legare P, Bernhardt P, Leuvrey C, Rogez G (2006) Chem Mater 18:4399–4404
Tang J, Myers M, Bosnick KA, Brus LE (2003) J Phys Chem B 107:7501–7506
Chamritski I, Burns G (2005) J Phys Chem B 109:4965–4968
Zakharova IN, Shipilin MA, Alekseev VP, Shipilin AM (2012) Tech Phys Lett 38(1):55–58 (ISSN 1063_7850)
Iyengar SJ, Joy M, Ghosh CK, Dey S, Kotnalad RK, Ghosha S (2014) RSC Adv 4:64919–64929
Ozdemiar O, Dunlop DJ, Moskowitz BM (1993) Geophys Res Lett 20(16):1671–1674
El Mendili Y, Grasset F, Randrianantoandro N, Nerambourg N, Greneche JM, Bardeau JF (2015) J Phys Chem C 119:10662–10668
Santos JG, Silveira LB, Fegueredo PHS, Araújo BF, Peternele WS, Rodriguez AFR, Vilela EC, Garg VK, Oliveira AC, Azevedo RB, Morais PC (2012) J Nanosci Nanotechnol 12:1–5
Pati SS, Singh LH, Guimaraes EM, Mantilla J, Coaquira JAH, Oliveira AC, Sharma VK, Garg VK (2016) J Alloys Compd 684(5):68–74
Klencsár Z, Kuzmann E, Vértes A (1996) J Radional Chem Lett 210:105
Oliveira AC, Tronconi AL, Buske N, Morais PC (2002) J Magn Magn Mater 252:56
Laurent S, Mahmoudi M (2011) Int J Mol Epidemiol Genet 2(4):367–390
Wasilewski P, Günther K (1999) Geophys Res Lett 26(15):2275–2278
Kuzmann E, Nagy S, Vértes A, Weiszburg TG, Garg VK (1998) In: Vertes A, Nagy S, Süvegh K (eds) Geological and mineralogical applications of Mössbauer spectroscopy in Nuclear Methods in Mineralogy and Geology: Techniques and Applications. Plenum Press, New York, pp 285–376
Rümenapp C, Wagner FE, Gleich B (2015) J Magn Magn Mater 380:241–245
Hesse J, Rübartsch A (1974) J Phys E 7:526
da Silva SW, Nakagomi F, Silva MS, Franco A Jr, Garg VK, Oliveira AC, Morais PC (2012) J Nanopart Res 14:798
Shebanova ON, Lazor P (2003) J Solid State Chem 174:424–430
Wohlfarth EP (1980) Ferromagnetic materials: a handbook on the properties of magnetically ordered substances. Elsevier, Amsterdam
He YP, Miao YM, Li CR, Wang SQ, Cao L, Xie SS, Yang GZ, Zou BS, Bruda C (2005) Phys Rev B 71:125411
Vargas H, Miranda LCM (1988) Phys Rep 161:43
Frison R, Cernuto G, Cervellino A, Zaharko O, Colonna GM, Guagliardi A, Masciocchi N (2013) Chem Mater 25:4820–4827
Mérela DS, Doa MLT, Gaillarda S, Dupaud P, Renauda JL (2015) Coord Chem Rev 288:50–68
Cornell RM, Schwertmann U (2006) The iron oxides: structure, properties, reactions, Occurrences and Uses. Wiley, Cambridge
McEntee M, Tang W, Neurock M, Yates JT Jr (2015) ACS Catal 5:744–753
Acknowledgements
This work was supported by grants of CAPES (No A127/2013) and OTKA (No K115913 and K115784). This work was carried out within the Agreement of Cooperation between Eötvös Loránd University (Budapest) and Universidade de Brasília (Brasília).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, S.W., Guilherme, L.R., de Oliveira, A.C. et al. Mössbauer and Raman spectroscopic study of oxidation and reduction of iron oxide nanoparticles promoted by various carboxylic acid layers. J Radioanal Nucl Chem 312, 111–119 (2017). https://doi.org/10.1007/s10967-017-5195-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5195-0