Abstract
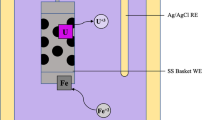
Recovery of metallic uranium has been achieved by electrolytic reduction of uranium oxide in a molten LiCl–Li2O electrolyte at 650 °C, followed by the removal of the residual salt by vacuum distillation at 850 °C. Four types of stainless steel mesh baskets, with various mesh sizes (325, 1,400 and 2,300 meshes) and either three or five ply layers, were used both as cathodes and to contain the reduced product in the distillation stage. The recovered uranium had a metal fraction greater than 98.8 % and contained no residual salt.











Similar content being viewed by others
References
Laidler JJ, Battles JE, Miller WE, Ackerman JP, Carls EL (1997) Prog Nucl Energy 31:131–140
Benedict RW, McFarlane HF (1998) Radwaste Mag 5:23
Iizuka M, Sakamura Y, Inoue T (2008) Nucl Eng Technol 40:183–190
Simpson MF, Herrmann SD (2008) Nucl Technol 162:179–183
Yoo JH, Seo CS, Kim EH, Lee H (2008) Nucl Eng Technol 40:581–592
Kitawaki S, Shinozaki T, Fukushima M, Usami T, Yahagi N, Kurata M (2008) Nucl Technol 162:118–123
Serp J, Konings RJM, Malmbeck R, Rebizant J, Scheppler C, Glatz JP (2004) J Electroanal Chem 561:143–148
Willit JL, Miller WE, Battles JE (1992) J Nucl Mater 195:229–249
Jeong SM, Park SB, Hong SS, Seo CS, Park SW (2006) J Radioanal Nucl Chem 268:349–356
Park SB, Park BH, Jeong SM, Hur JM, Seo CS, Choi SH, Park SW (2006) J Radioanal Nucl Chem 268:489–495
Goff KM, Wass JC, Marsden KC, Teske GM (2011) Nucl Eng Technol 43:335–342
Koyama T, Sakamura Y, Ogata T, Kobayashi H (2009) Proc Global 2009 pp 9161 Paris, France
Hur JM, Kim TJ, Choi IK, Do JB, Hong SS, Seo CS (2008) Nucl Technol 162:192–198
Herrmann SD, Li SX (2010) Nucl Technol 171:247–265
Sakamura Y, Omori T (2010) Nucl Technol 171:266–275
Jeong SM, Shin HS, Hong SS, Hur JM, Do JB, Lee HS (2010) Electrochim Acta 55:1749–1755
Usami T, Kurata M, Inoue T, Sims HE, Beetham SA, Jenkins JA (2002) J Nucl Mater 300:15–26
Hur JM, Seo CS, Hong SS, Kang DS, Park SW (2003) React Kinet Catal Lett 80:217–222
Chen GZ, Fray DJ, Farthing TW (2000) Nature 407:361–364
Sakamura Y, Kurata M, Inoue T (2006) J Electrochem Soc 153:D31–D39
Sakamura Y, Omori T, Inoue T (2008) Nucl Technol 162:169–178
Hur JH, Jeong SM, Lee H (2010) Electrochem Commun 12:706–709
Gourishankar KV, Redey L, Williamson M (2002) In: Schneider WA (ed) Light Metals. The Minerals, Metals and Materials Society, USA (p. 1075)
Redey L, Gourishankar KL (2003) US Patent 6540902
Herrmann SD, Li SX, Simpson MF, Phongikarroon S (2006) Sep Sci Technol 41:1965–1983
Herrmann SD, Li SX, Serrano-Rodriguez EBE (2009) Proc Global 9059
Choi EY, Kim JK, Im HS, Choi IK, Na SH, Lee JW, Jeong SM, Hur JM (2013) J Nucl Mater 437:178–187
Kim IS, Oh SC, Im HS, Hur JM, Lee HS (2013) J Radioanal Nucl Chem 296:1413–1417
Chapman LR, Holcombe CE (1984) J Nucl Mater 126:323–326
Leibowitz L, Blomquist RA (1991) J Nucl Mater 184:47–52
Huang K, Park Y, Ewh A, Sencer BH, Kennedy JR, Coffey KR, Sohn YH (2012) J Nucl Mater 424:82–88
Rai AK, Raju S, Vijayalakshmi M (2013) J Nucl Mater 432:520–528
Acknowledgments
This work was supported by the Nuclear Research & Development Program of the National Research Foundation (NRF), in a grant funded by the Korean Government.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Choi, EY., Won, C.Y., Kang, DS. et al. Production of uranium metal via electrolytic reduction of uranium oxide in molten LiCl and salt distillation. J Radioanal Nucl Chem 304, 535–546 (2015). https://doi.org/10.1007/s10967-014-3842-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3842-2