Abstract
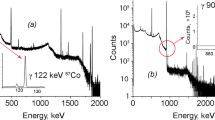
The kinetics of uranium, plutonium and americium electrodeposition on steel targets from organic solutions of diphenyl-(N,N-dibutyl) carbamoylmethylphosphine oxide supplemented with the ionic liquid of trihexyltetradecylphosphonium hexafluorophosphate ([PH4]+PF6 −) in ethanol or N,N-dimethylformamide has been studied. When running a process in a dimethylformamide medium, and 0.05 M concentration of [PH4]+PF6 − americium deposition degree exceeds 95 % at the electrolysis time of 2 h. Alpha-spectra resolution of obtained target does not exceed 40 keV.




Similar content being viewed by others
References
Novikov AP, Myasoedov BF (2003) Radiochemical procedures for speciation of actinides in the environment. Environment protection against radioactive pollution. 147–154
Novikov AP, Pribylova GA, Smirnov IV The method of electrochemical actinide sedimentation. RF patent no 2493295 dated 13.07.2012
Giridhar P, Venkatesan KA, Subramaniam S, Srinivasan TG, Vasudeva Rao PR (2008) Extraction of uranium (YI) by 1.1 M tri-n-butylphosphate/ionic liquid and the feasibility of recovery by direct electrodeposition from organic phase. J Alloys and Compounds 448:104–108
Asanuma N, Harada M, Yasuike Y, Nogami M, Suzuki K, Ikeda Y (2007) Electrolytic reduction of spent light water reactor fuel bench-scale experiment results. J Nucl Sci Technol 44(3):368–372
Dai S, Ju YH, Barnes CE (1999) Solvent extraction of strontium nitrate by a crown ether using room-temperature ionic liquids. J Chem Soc, Dalton Trans 21:1201–1202
Visser AN, Swatloski RP, Reichert WM, Griffin ST, Rogers RD (2000) Traditional extractants in nontraditional solvents: groups 1 and 2 extraction by crown ethers in room-temperature ionic liquids. Ind Eng Chem Res 39:3596–3604
Karavan M, Arnaud-Neu F, Hubscher-Bruder V, Smirnov IV, Kalchenko V (2009) Novel phosphorylated calixarenes for the recognition of f-elements. J Incl Phenom Macrocycl Chem 23:200–210
Gutowski KE, Cocalia VA, Griffin ST, Bridges NJ, Dixon DA, Rogers RD (2007) Interactions of 1-methylimidazole with UO2(CH3CO2)2 and UO2(NO3)2: structural, spectroscopic, and theoretical evidence for imidazole binding to the uranyl ion. J Am Chem Soc 129:526–536
Fraga-Dubreuil J, Bazureau JP (2003) Efficient combination of task-specific ionic liquid and microwave dielectric heating applied to one-pot three component synthesis of a small library of 4-thiazolidinones. Tetrahedron 59:6121–6130
Litvina MN, Chmutova MK, Myasoyedov BF, Kabanchik MI (1996) Extraction of rare earth metals and americium and their division factors in the nitric acid—oxydes of diaryl-(dialkyl)-[dialkylcarbamoylmethyl]-phosphines. Radiochemistry 38:525–530
Pribylova GA, Smirnov IV, Novikov AP (2013) Effect of ionic liquids on the extraction of americium by diphenyl (dibutyl) carbamoylmethylphosphine oxide in dichloroethane from nitric acid solutions. J Radioanal Nucl Chem 295:83–87
Pletnev IV, Smirnov SV, Khachatrjan KS, Zernov VV (2004) Use of ionic liquids in extraction. Russ J Chem 48:51–58
Bhatt AI, May I, Volkovich VA, Hetherington ME, Lewin B, Thied RC, Ertok N (2002) Group 15 quaternary alkyl bistriflimides: ionic liquids with potential application in electropositive metal deposition and as supporting electrolytes. J Chem Soc Dalton Trans 24:4532–4534
Bhatt AI, May I, Volkovich VA (2005) Structural characterization of a lanthanium bistriflimide complex, La(N(SO2CF3)2)3(H2O)3, and an investigation of La, Sm and Eu electrochemistry in a room-temperature ionic liquids, [Me3N17Bu]N(SO2CF3)2]. Inorg Chem 44:4934–4940
Bhatt AI, Duffy NW, Collison D, May I, Lewin RG (2006) Cyclic voltammetry of Th(IY) in the room-temperature ionic liquid [Me3N17Bu][N(SO2CF3)2]. Inorg Chem 45:1677–1682
Acknowledgments
This work was performed under the Russian Basic Research Foundation support (14-05-00181).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Novikov, A.P., Ryleeva, V.S., Abramova, A.V. et al. Electrodeposition of americium on the stainless steel support for the purpose of radiochemical assay. J Radioanal Nucl Chem 302, 543–547 (2014). https://doi.org/10.1007/s10967-014-3246-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3246-3