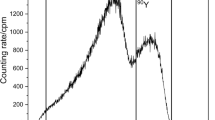
Abstract
A novel direct method for the determination of EDTA in alkaline radioactive evaporator residue solution was developed and validated based on ion chromatography with suppressed conductimetric detection and anion exchange columns (A Supp 4, 4 mm × 250 mm and A Supp 5, 4 mm × 150 mm). The yttrium-EDTA complex resulted one single chromatographic peak in the eluent and allowed the correct determination of EDTA in an alkaline, high concentration radioactive waste water. Depending on coexisting substances, suitable eluent is 10.0 mM carbonate buffer/pH 10.6 or 10.75 (t R,Y–EDTA = 7.01 and 6.4 min, respectively). For 10.0 mM carbonate buffer/pH 10.6 and isocratic flow rate of 1.0 cm3/min, a linear calibration curve was obtained from 5 to 40 mg/dm3 (r > 0.999) EDTA. Good resolution was achieved from commonly coexisting anions (chloride, nitrite, nitrate, sulphate, phosphate, bromide and citrate). The developed simple ion chromatographic method was applied for the assay of EDTA in various radioactive alkaline solutions.










Similar content being viewed by others
References
Besunder JB, Super DM, Anderson RL (1997) Comparison of dimercaptosuccinic acid and calcium disodium ethylenediaminetetraacetic acid versus dimercaptopropanol and ethylenediaminetetraacetic acid in children with lead poisoning. J Pediatr 130(6):966–971
Deacon M, Smyth MR, Th Tuinstra LGM (1994) Chromatographic separations of metal chelates present in commercial fertilizers. II. Development of an ion-pair chromatographic separation for the simultaneous determination of the Fe(III) chelates of EDTA, DTPA, HEEDTA, EDDHA and EDDHMA and the Cu (II), Zn (II) and Mn(II) chetales of EDTA. J Chromatogr A 657:69–76
Sillanpaa M, Sihvonen ML (1997) Analysis of EDTA and DTPA. Talanta 44(8):1487–1497
Clinckemaille GG (1968) Determination of nitrilotriacetic acid and ethylenediaminetetra-acetic acid in granular detergent formulations client. Anal Chim Acta 43:520–522
Hamano T, Mistuhashi Y, Kojima N, Aoki N, Shibata M, Ito Y, Oji Y (1993) Sensitive spectrophotometric method for the determination of ethylenediaminetetraacetic acid in foods. Analyst 118:909–912
Nomura T, Nakagawa G (1980) Tensammetric determination of microgram amounts of EDTA using a catalytic oxidation of 2-amino-4-methylphenol with vanadium. J Electroanal Chem 111:319–324
Stolzberg RJ (1977) Potential inaccuracy in trace metal speciation measurements by differential pulse polarography. Anal Chim Acta 92(1):193–196
Hadjiioannou TP, Koupparis MA, Efstathiou CE (1977) Evaluation of a perchlorate-selective electrode for catalytic titrations involving periodate indicator reactions. Anal Chim Acta 88(2):281–287
Stojek Z, Osteryoung J (1981) Direct determination of chelons at trace levels by one-drop square-wave polarography. Anal Chem 53(6):847–851
Voulgaropoulos A, Tzivanakis N (1992) Use of ion exchangers for the voltammetric determination of NTA and EDTA in natural waters. Electroanalysis 4(6):647–651
Fogg AG, Fernandez-Arciniega MA, Alonso RM (1985) Flow injection amperometric determination of nitroprusside at a glassy carbon electrode and at a sessile mercury drop electrode. Analyst 110:1201–1204
Pozdniakova S, Ragauskas R, Dikcius A, Padarauskas A (1999) Determination of EDTA in used fixing solutions by capillary electrophoresis. Fresenius J Anal Chem 363:124–125
Dhara S, Sarkar S, Basu S, Chattopadhyay P, (2009) Separation of the 90Sr–90Y pair with cerium(IV) iodotungstate cation exchanger. Appl Radiat Isot 67:530–534
Chakraborty R, Chattopadhyay P (2012) Sodium titaniumsilicate as ion exchanger: synthesis, characterization and application in separation of 90Y from 90Sr. J Radioanal Nucl Chem 294:31–35
Krokidis AA et al (1999) EDTA determination in pharmaceutical formulations and canned foods based on ion chromatography with suppressed conductimetric detection. Anal Chim Acta 535:57–63
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pátzay, G., Ramadan, Y. & Tonkó, C. New EDTA determination method based on ion chromatography with suppressed conductimetric detection. J Radioanal Nucl Chem 302, 161–168 (2014). https://doi.org/10.1007/s10967-014-3238-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3238-3