Abstract
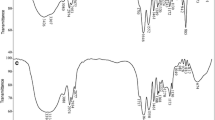
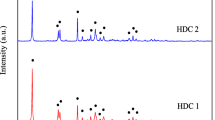
Unmodified and iron-modified hydrogels were synthesized from polyvinyl alcohol and polyvinylpyrrolidone; these materials were used for the adsorption of fluoride ions from an aqueous solution. The structural and morphological characteristics of the unmodified (HG) and modified (HG-Fe) hydrogels were determined by means of hydration percentage, point of zero charge (PZC), infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and dispersive energy spectroscopy (EDS). The parameters considered in the adsorption processes were contact time, pH, fluoride concentration and dosage. The kinetic data were obtained at an initial pH of 6.7 and were best fitted to the Elovich model (R2 = 0.994); the adsorption capacity was higher for HG-Fe than HG. The data obtained at pH 5 were adjusted to the Lagergren equation indicating physical adsorption. The capacities of both materials (HG and HG-Fe) were similar, and the equilibrium time was shorter for HG than HG-Fe. The isotherms were best fitted to the Langmuir model, indicating homogeneous materials. The maximum adsorption capacities were similar for both adsorbents (HG-Fe and HG), and the thermodynamic parameters showed that the processes are exothermic.









Similar content being viewed by others
Data availability
Data will be made available on request.
References
Perumal S, Atchudan R, Edison T, Suresh R, Karpagavinayagam P, Vedhi C (2021) Short review on recent advances of hydrogel-based adsorbents for heavy metal ions. Metals 11(6):864. https://doi.org/10.3390/met11060864
Akhtar M, Hanif M, Ranjha N (2015) Methods of synthesis of hydrogels. A review. Saudi Pharma J 24. https://doi.org/10.1016/j.jsps.2015.03.022
Ding QL, Wu ZX, Tao K, Wei YM, Wang WY, Yang BR, Xie X, Wu J (2022) Environment tolerant, adaptable and stretchable organohydrogels: Preparation, optimization, and applications. Mater Horiz 9. https://doi.org/10.1039/d1mh01871j
Rivera-Hernández G, Antunes-Ricardo M, Martínez-Morales P, Sánchez ML (2021) Polyvinyl alcohol based-drug delivery systems for cancer treatment. Int J Pharm 600:120478. https://doi.org/10.1016/j.ijpharm.2021.120478
Meireles D, Boggione IJ, Menezes de Souza S, Santos RC (2021) Preparation of polyvinyl alcohol hydrogel containing bacteriophage and its evaluation for potential use in the healing of skin wounds. J Drug Delivery Sci Technol 63. https://doi.org/10.1016/j.jddst.2021.102484
Zulfiqar M, Lee SY, Mafize AA, Mastura NA, Johari K, Ekmi N (2020) Efficient removal of Pb(II) from aqueous solutions by using oil palm bio-waste/MWCNTs reinforced pva hydrogel composites: kinetic, isotherm and thermodynamic modeling. Polymers 12(2). https://doi.org/10.3390/polym12020430
Aljar M, Rashdan S, Abd A (2021) Environmentally friendly polyvinyl alcohol – alginate/bentonite nanocomposite hydrogel beads as efficient adsorbents for removal of toxic Methylene Blue from Aqueous Solution. Polymers 13(22):4000. https://doi.org/10.3390/polym13224000
Todros S, Barbon S, Stocco E, Favaron M, Macchi V, De Caro R, Porzionato A, Pavan PG (2022) Time-dependent mechanical behavior of partially oxidized polyvinyl alcohol hydrogels for tissue engineering. J Mech Behav Biomed Mater 125:104966. https://doi.org/10.1016/j.jmbbm.2021.104966
Kumar A, Han SS (2017) PVA-based hydrogels for tissue engineering: a review. Int J Polym Mater Polym Biomater 66:159. https://doi.org/10.1080/00914037.2016.1190930
Musgrave CS, Fang F (2019) Contact lens materials: a materials science perspective. Materials 12(2):261. https://doi.org/10.3390/ma12020261
Kharaghani D, Dutta D, Gitigard P, Tamada Y, Katagiri A, Phan DN, Willcox MD, Kim IS (2019) Development of antibacterial contact lenses containing metallic nanoparticles. Polym Test 79. https://doi.org/10.1016/j.polymertesting.2019.106034
Xin M, Li J, Ma Z, Pan L, Shi Y (2020) MXenes and their applications in wearable sensors. Front Chem 8:297. https://doi.org/10.3389/fchem.2020.00297
Bae J, Park J, Kim S, Cho H, Kim HJ, Park S, Shin DS (2020) Tailored hydrogels for biosensor applications. J Ind Eng Chem. https://doi.org/10.1016/j.jiec.2020.05.001
Ghane N, Beigi MH, Labbaf S, Nasr-Esfahani MH, Kiani A (2020) Design of hydrogel-based scaffolds for the treatment of spinal cord injuries. J Mater Chem B. https://doi.org/10.1039/d0tb01842b
Fard HN, Pour GB, Sarvi MN (2019) PVA-based supercapacitors. Ionics 25:2951. https://doi.org/10.1007/s11581-019-03048-8
Warkar SG, Kumar A (2019) Synthesis and assessment of carboxymethyl tamarind kernel gum based novel superabsorbent hydrogels for agricultural applications. Polymer 182:121823. https://doi.org/10.1016/j.polymer.2019.121823
Sumer T, Bakar A, Niaz M, Amir A, Bakar A, Al-Amiery AA (2015) Review properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 20. https://doi.org/10.3390/molecules201219884
Baker MI, Walsh SP, Schwartz Z, Boyan B (2012) A review of polyvinyl alcohol and its uses in cartilage and orthopedic applications. J Biomed Mater Res Part B 100. https://doi.org/10.1002/jbm.b.32694
Teodorescu M, Bercea M (2015) Poly(vinylpyrrolidone) - a versatile polymer for Biomedical and Beyond Medical Applications. Polym Plast Technol Eng 54:923. https://doi.org/10.1080/03602559.2014.979506
Park KR, Nho YC (2004) Preparation and characterization by radiation of hydrogels of PVA and PVP containing Aloe vera. J Appl Polym Sci 91(3):1612. https://doi.org/10.1002/app.13299
Husain MS, Gupta A, Alashwal BY, Sharma S (2018) Synthesis of PVA/PVP based hydrogel for biomedical applications: a review. Energy Sources Part A 40. https://doi.org/10.1080/15567036.2018.1495786
Cassu SN, Felisberti MI (1997) Poly(vinyl alcohol) and poly(vinyl pyrrolidone) blends: miscibility, microheterogeneity and free volume change. Polymer 38:3907. https://doi.org/10.1016/s0032-3861(96)00959-7
Morariu S, Bercea M, Teodorescu M, Avadanei M (2016) Tailoring the properties of poly(vinyl alcohol)/poly(vinylpyrrolidone) hydrogels for biomedical applications. Eur Polym J 84. https://doi.org/10.1016/j.eurpolymj.2016.09.033
Rahaman MS, Hasnine SM, Ahmed T, Sultana S, Quaiyum MD, Serajum M, Ullah N, Kumar S, Nazmul M, Sahadat M, Chandra N (2021) Radiation crosslinked polyvinyl alcohol/polyvinyl pyrrolidone/acrylic acid hydrogels: swelling, crosslinking, and dye adsorption study. Iran Polym J 30. https://doi.org/10.1007/s13726-021-00949-2
Tassanapukdee Y, Prayongpan P, Songsrirote K (2021) Removal of heavy metal ions from an aqueous solution by CS/PVA/PVP composite hydrogel synthesized using microwaved-assisted irradiation. Environ Technol Innov 24:101898. https://doi.org/10.1016/j.eti.2021.101898
Amiri S, Asghari A, Vatanpour V, Rajabi M (2020) Fabrication and characterization of a novel polyvinyl alcohol-graphene oxide-sodium alginate nanocomposite hydrogel blended PES nanofiltration membrane for improved water purification. Sep Purif Technol 250:117216. https://doi.org/10.1016/j.seppur.2020.117216
D’Amelia R, Mancuso J (2020) The study of polyvinyl pyrrolidone-polyvinyl alcohol copolymers and blends. J Polym Biopolym Phys Chem 8. https://doi.org/10.12691/jpbpc-8-1-1
Ma S, Hou JJ, Yang H, Xu Z (2017) Preparation of renewable porous TiO2/PVA composite sphere as photocatalyst for methyl orange degradation. J Porous Mater 25. https://doi.org/10.1007/s10934-017-0518-7
Mukherjee D, Barghi S, Ray AK (2014) Degradation of methyl orange by TiO2/polymeric film photocatalyst. Can J Chem Eng 92:1661. https://doi.org/10.1002/cjce.22028
Mallakpour S, Motirasoul F (2018) Ultrasonication synthesis of PVA/PVP/α-MnO2 -stearic acid blend nanocomposites for adsorbing cd II ion. Ultrason Sonochem 40:410. https://doi.org/10.1016/j.ultsonch.2017.07.034
Jabli M, Hamdaoui M, Jabli A, Ghandour Y, Ben B (2014) A comparative study on the performance of dye removal, from aqueous suspension, using (2-hydroxypropyl)-β-cyclodextrin-CS, PVP-PVA-CS, PVA-CS, PVP-CS and plain CS microspheres. J Text Inst 105. https://doi.org/10.1080/00405000.2013.843851
Ahmad S, Singh R, Arfin T, Neeti K (2022) Fluoride contamination, consequences and removal techniques in water: a review. Environ Sci Adv 5. https://doi.org/10.1039/d1va00039j
Sobeih MM, Shahat MF, Osman A, Zaid MA, Nassar MY (2020) Glauconite clay-functionalized chitosan nanocomposites for efficient adsorptive removal of Fluoride ions from polluted aqueous solutions. RSC Adv 10. https://doi.org/10.1039/d0ra02340j
Meilani V, Lee JI, Kang JK, Lee CG, Jeong S, Park SJ (2021) Application of aluminum-modified food waste biochar as adsorbent of fluoride in aqueous solutions and optimization of production using response surface methodology. Microporous Mesoporous Mater 312:110764. https://doi.org/10.1016/j.micromeso.2020.110764
Jeyaseelan A, Naushad M, Ahamad T, Viswanathan N (2021) Design and development of amine functionalized iron-based metal organic frameworks for selective fluoride removal from water environment. J Environ Chem Eng 9:104563. https://doi.org/10.1016/j.jece.2020.104563
Annan E, Nyankson E, Agyei-Tuffour B, Kofi E, Nkrumah G, Modupeh JA, Oteng-Peprah M (2021) Synthesis and characterization of modified Kaolin-Bentonite composites for enhanced fluoride removal from drinking Water. Adv Mater Sci Eng. https://doi.org/10.1155/2021/6679422
Zhou J, Yu J, Liao H, Zhang Y, Lou X (2020) Facile fabrication of bimetallic collagen fiber particles via immobilizing zirconium on chrome-tanned leather as adsorbent for fluoride removal from ground water near hot spring. Sep Sci Technol. https://doi.org/10.1080/01496395.2019.1574826
Tolkou AK, Manousi N, Zachariadis GA, Deliyanni EA (2021) Recently developed adsorbing materials for fluoride removal from water and fluoride analytical determination techniques: a review. Sustainability 13:7061. https://doi.org/10.3390/su13137061
Elewa AM, Amer AA, Attallah MF, Gad HA, Mohamed ZA, Ahmed IA (2023) Chemically activated carbon based on biomass for adsorption of Fe(III) and mn(II) ions from aqueous solution. Materials. https://doi.org/10.3390/ma16031251
Gutiérrez M, Alarcon-Herrera MT, Gaytan-Alarcon AP (2023) Arsenic and fluorine in groundwater in northern Mexico: spatial distribution and enrichment factors. Environ Monit Assess 195:212. https://doi.org/10.1007/s10661-022-10818-x
Abd EM, Ghanem HL (2009) Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. J Polym Res 16. https://doi.org/10.1007/s10965-008-9196-0
Razzak MT, Darwis D, Zainuddin S (2001) Irradiation of polyvinyl alcohol and polyvinyl pyrrolidone blended hydrogel for wound dressing. Radiat Phys Chem 62(1):107. https://doi.org/10.1016/s0969-806x(01)00427-3
Fabryanty R, Valencia C, Soetaredjo FE, Nyoo J, Permatasari S, Kurniawan A, Ju YH, Ismadaji S (2017) Removal of crystal violet dye by adsorption using bentonite - alginate composite. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2017.10.057
Zhu F, Guo Z, Hu X (2020) Fluoride removal efficiencies and mechanism of schwertmannite from KMnO4/MnO2–Fe(II) processes. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.12278
Moghaddama RH, Dadfarnia S, Shabani AMH, Tavakol M (2018) Synthesis of composite hydrogel of glutamic acid, gum tragacanth, and anionic polyacrylamide by electron beam irradiation for uranium (VI) removal from aqueous samples: equilibrium, kinetics, and thermodynamic studies. Carbohydr Polym. https://doi.org/10.1016/j.carbpol.2018.10.030
Singh S, Basu H, Bassan MKT, Kumar R (2022) Thiol functionalised silica microsphere loaded polymeric hydrogel: development of a novel hybrid sorbent for removal of lead and cadmium. Chemosphere. https://doi.org/10.1016/j.chemosphere.2021.131659
Xu D, Lei F, Chen H, Yin L, Xie J (2019) One-step hydrothermal synthesis and optical properties of self-quenching-resistant carbon dots towards fluorescent ink and as nanosensors for Fe3+ detection. RSC Adv. https://doi.org/10.1039/c8ra10570g
Cao L, Wu X, Wang Q, Wang J (2018) Biocompatible nanocomposite of TiO2 incorporated bi-polymer for articular cartilage tissue regeneration: a facile material. J Photochem Photobiol B 178:440. https://doi.org/10.1016/j.jphotobiol.2017.10.026
Moreno-Marcelino JE, Gutierrez-Segura E, Vilchis-Nestor AR, Castro-Longoria E, López-Téllez G (2020) Shape memory hybrid based on polyvinyl alcohol and 0D silver nanoparticles. Polym Test 90:106668. https://doi.org/10.1016/j.polymertesting.2020.106668
Wu YH, Yu DG, Li HP, Wu XY, Li XY (2017) Medicated structural PVP/PEG composites fabricated using coaxial electrospinning. e-Polymers. https://doi.org/10.1515/epoly-2016-0244
Zidan HM, Abdelrazek EM, Abdelghany AM, Taeabiah AE (2018) Characterization and some physical studies of PVA/PVP filled with MWCNTs. J Mater Res Technol. https://doi.org/10.1016/j.jmrt.2018.04.023
Daems N, Milis S, Verbeke R, Szymczyk A, Pescarmona P, Vankelecom IFJ (2018) High-performance membranes with full pH-stability. RSC Adv. https://doi.org/10.1039/c7ra13663c
Sarip MN, Noor MFH, Ahmad Z, Shuhaime N, Dahan RM, Arshad AN, Ismail WINW (2018) Conductivity study of polyvinyl alcohol/polyvinyl pyrrolidone (PVA/PVP)-KOH coatings system. https://doi.org/10.1063/1.5066978
Wang B, Zhang Q, Xiong G (2019) Bakelite-type anionic microporous organic polymers with high capacity for selective adsorption of cationic dyes from water. Chem Eng J 36. https://doi.org/10.1016/j.cej.2019.02.089
Bhattacharyya R, Ray SK (2013) Kinetic and equilibrium modeling for adsorption of textile dyes in aqueous solutions by carboxymethyl cellulose/poly(acrylamide-co-hydroxyethyl methacrylate) semiinterpenetrating network hydrogel. Polym Eng Sci. https://doi.org/10.1002/pen.23501
Lee J, Hong S, Lee C, Park S (2021) Fluoride removal by thermally treated eggshells with high adsorption capacity, low cost, and easy acquisition. Environ Sci Pollut Res 28. https://doi.org/10.1007/s11356-021-13284-z
Shanika M, Wimalasiri AK, Dziemidowicz K, Williams G, Dissanayake DP, Nalin de Silva KM, De Silva R (2021) Biopolymer based nanohydroxyapatite composites for the removal of fluoride, lead, cadmium, and arsenic from water. ACS Omega 6. https://doi.org/10.1021/acsomega.1c00316
Everaert M, Bergmans J, Broos K, Hermans B, Michielsen B (2021) Granulation and calcination of alum sludge for the development of a phosphorus adsorbent: from lab scale to pilot scale. J Environ Manage 279:111525. https://doi.org/10.1016/j.jenvman.2020.111525
Huang L, Yang Z, Li X, Hou L, Alhassan S, Wang H (2020) Synthesis of hierarchical hollow MIL-53(Al)-NH2 as an adsorbent for removing fluoride: experimental and theoretical perspective. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-10975-x
Sengupta P, Saha S, Banerjee S, Dey A, Sarkar P (2020) Removal of fluoride ion from drinking water by a new Fe(OH)3 / nano CaO impregnated chitosan composite adsorbent. Polym-Plast Technol Mater. https://doi.org/10.1080/25740881.2020.1725567
Aigbe UO, Onyancha RB, Ukhurebor KE, Onyebuchi K (2020) Removal of fluoride ions using a polypyrrole magnetic nanocomposite influenced by a rotating magnetic field. RSC Adv 10. https://doi.org/10.1039/c9ra07379e
Rajkumar S, Murugesh S, Sivasankar V, Chaabane T (2015) Low-cost fluoride adsorbents prepared from a renewable biowaste: syntheses, characterization and modeling studies. Arab J Chem. https://doi.org/10.1016/j.arabjc.2015.06.028
Dong S, Wang Y (2016) Characterization and adsorption properties of a lanthanum-loaded magnetic cationic hydrogel composite for fluoride removal. Water Res 88:852. https://doi.org/10.1016/j.watres.2015.11.013
Bazrafshan E, Balarak D, Panahi AH, Mahvi A (2016) Fluoride removal from Aqueous solutions by Cupric Oxide nanoparticles. Fluoride 49. https://doi.org/10.13140/RG.2.2.33178.90564
Liu Q, Zhang L, Yang B, Huang R (2015) Removal of fluoride from aqueous solution using zr(IV) immobilized cross-linked chitosan. Int J Biol Macromol 77:15. https://doi.org/10.1016/j.ijbiomac.2015.03.008
Teutli-Sequeira A, Solache-Ríos M, Martínez-Miranda V, Linares-Hernández I (2014) Comparison of aluminum modified natural materials in the removal of fluoride ions. J Colloid Interface Sci 418:254. https://doi.org/10.1016/j.jcis.2013.12.020
Singh KP, Shyam Kumar A, Paniteja M, Singh S (2019) Novel properties of Epipremnum aureum for treatment of fluoride-contaminated water. SN Appl Sci 1(7):741. https://doi.org/10.1007/s42452-019-0773-0
Tomar V, Surendra P, Kumar D (2013) Adsorptive removal of fluoride from aqueous media using citrus limonum (lemon) leaf. Microchem J. https://doi.org/10.1016/j.microc.2013.09.010
Farah A, Farah N, Talpur AB, Muhammad AS, Muhammad AB (2015) Biosorption of fluoride from aqueous solution by white—rot fungus Pleurotus Eryngii ATCC 90888. Environ Nanotechnol Monit Manag. https://doi.org/10.1016/j.enmm.2014.11.003
Sivasankar V, Rajkumar S, Murugesh S, Darchen A (2012) Tamarind (Tamarindus indica) fruit shell carbon: a calcium-rich promisi,ng adsorbent for fluoride removal from groundwater. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2012.05.015
Ma Y, Shi F, Zheng X, Ma J, Gao C (2011) Removal of fluoride from aqueous solution using granular acid-treated bentonite (GHB): batch and column studies. J Hazard Mater 185:1073. https://doi.org/10.1016/j.jhazmat.2010.10.016
Teutli-Sequeira A, Martínez-Miranda V, Solache-Ríos M, Linares-Hernández I (2013) Aluminum and lanthanum effects in natural materials on the adsorption of fluoride ions. J Fluor Chem. https://doi.org/10.1016/j.jfluchem.2013.01.015
Lv L, He J, Wei M, Evans DG, Duan X (2006) Factors influencing the removal of fluoride from aqueous solution by calcined Mg-Al-CO3 layered double hydroxides. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2005.10.01
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361. https://doi.org/10.1021/ja02242a004
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Eng J 156. https://doi.org/10.1016/j.cej.2009.09.013
Giles CH, Smith D, Huitson A (1974) A general treatment and classification of the solute adsorption isotherm. J Colloid Interface Sci. https://doi.org/10.1016/0021-9797(74)90252-5
Sipos P (2021) Searching for optimum adsorption curve for metal sorption on soils: comparison of various isotherm models fitted by different error functions. SN Appl Sci 3:387. https://doi.org/10.1007/s42452-021-04383-0
Alaqarbeh M (2021) Adsorption phenomena: definition, mechanisms, and adsorption types: short review. Green and Applied Chemistry. https://doi.org/10.48419/IMIST.PRSM/rhazes-v13.28283
Vieira T, Artifon SES, Cesco CT, Vilela PB, Becegato VA, Paulino AT (2020) Chitosan-based hydrogels for the sorption of metals and dyes in water: isothermal, kinetic, and thermodynamic evaluations. Colloid Polym Sci. https://doi.org/10.1007/s00396-020-04786-2
Eyube ES, Notani PP, Samaila H (2022) Analytical prediction of enthalpy and Gibbs free energy of gaseous molecules. Chem Thermodyn Therm Anal. https://doi.org/10.1016/j.ctta.2022.100060
Ebisike K, Okoronkwo AE, Alaneme KK, Akinribide O (2022) Thermodynamic study of the adsorption of Cd2+ and Ni2+ onto chitosan - silica hybrid aerogel from aqueous solution. Results Chem. https://doi.org/10.1016/j.rechem.2022.100730
Alver E, Metin AÛ, Brouers F (2020) Methylene blue adsorption on magnetic alginate/rice husk bio-composite. Int J Biol Macromol 154:104. https://doi.org/10.1016/j.ijbiomac.2020.02.330
Gu B, Schmitt J, Chen Z, Liang L, McCarthy F (1994) Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ Sci Technol. https://doi.org/10.1021/es00050a007
Zhang W, Zhou L, Tang H, Li H, Song W, Chen Z (2015) Modeling geochemical factors controlling fluoride concentration in groundwater. Arab J Geosci 8(11):9133. https://doi.org/10.1007/s12517-015-1933-1
Amro AN, Abhary MK, Shaikh MM, Ali S (2019) Removal of lead and cadmium ions from aqueous solution by adsorption on a low-cost Phragmites biomass. Processes 7:406. https://doi.org/10.3390/pr7070406
Alghamdi AA, Al-Odayni AB, Saeed WS, Al-Kahtani A, Alharti F, Aouak T (2019) Efficient adsorption of lead (II) from aqueous phase solutions using polypyrrole-based activated Carbon. Materials. https://doi.org/10.3390/ma12122020
Author information
Authors and Affiliations
Contributions
Conceptualization: V. Rosendo-González and E. Gutiérrez-Segura; data curation: V. Rosendo-González, E. Gutiérrez-Segura, and M. Solache-Rios; investigation: V. Rosendo-González and E. Gutiérrez-Segura; methodology: V. Rosendo-González, E. Gutiérrez-Segura, and M. Solache-Rios; writing—original draft: V. Rosendo-González and M. Solache-Rios; writing—review and editing: M.J.S.R., E. Gutiérrez-Segura and A. Amaya-Chavez.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rosendo-González, V., Gutiérrez-Segura, E., Solache-Rios, M. et al. Removal of fluoride ions from aqueous solutions on unmodified and iron-modified hydrogels. J Polym Res 31, 133 (2024). https://doi.org/10.1007/s10965-024-03954-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-024-03954-0