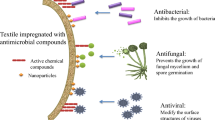
Abstract
We discuss here the preparation, characterization and antimicrobial properties of polyvinyl alcohol (PVA) based polymer films containing medicinally important plant extracts of Lawsonia inermis (henna) and Tamarindus indica (tamarind). The polymer films are prepared using a simple solution casting technique with concomitant heating at 42 °C. The ultraviolet-visible absorption spectrum and Fourier transform infrared spectroscopy (FTIR) of plant extracts confirm the presence of bioactive compounds, such as, 2-hydroxy, 1,4-naphthoquinone (lawsone) in henna extract and tannins, tartaric acid and reducing sugars in tamarind extract, respectively. FTIR analysis of the films confirms the presence of hydrogen bonding between PVA and tamarind phytochemicals in PVA-tamarind films. The surface topography and the average surface roughness analysis using an atomic force microscopy show that tamarind based polymer films have a lower surface roughness, due to a better interaction between PVA and tamarind phytochemicals, than that in henna based polymer films. Phase contrast microscopy images confirm the absence of polymer aggregation or pull-out in PVA-tamarind films, whereas, phase contrast microscopy images confirming the presence of such aggregation in PVA-henna films. Further, the thermal stability of the polymer films containing plant extract is studied in the temperature range of 30-250 °C. The antimicrobial activity of the films was studied using Kirby-Bauer Disc Diffusion method and the polymer films containing tamarind extract are found to exhibit antimicrobial activity against both E. coli and S. aureus. Quantitative analysis of the antimicrobial property of polymer films containing tamarind extract was carried-out using turbidimetry which further corroborates the antimicrobial property of these films. Thus, PVA-tamarind based films are promising candidates for antimicrobial applications, such as, in wound dressing as a ready to use bandage.











Similar content being viewed by others
Data availability
The data that support the findings of this study are available with the corresponding author.
References
Kebede T, Gadisa E, Tufa A (2021) Antimicrobial activities evaluation and phytochemical screening of some selected medicinal plants: A possible alternative in the treatment of multidrug-resistant microbes. PLoS ONE 16:e0249253
Monte J, Abreu AC, Borges A, Simoes LC, Simoes M (2014) Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens 3:473–498
Jadhav DY, Sahoo AK, Ghosh JS, Ranveer RC, Mali AM (2010) Phytochemical detection and in vitro evaluation of tamarind fruit pulp for potential antimicrobial activity. Int J Trop Med 5:68–72
Simoes M, Bennett RN, Rosa EAS (2009) Understanding antimicrobial activities of phytochemicals against multidrug resistant bacteria and biofilms. Nat Prod Rep 26:746–757
Khameneh B, Iranshahy M, Soheili V, Bazzaz BSF (2019) Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob Resist Infect Control 8:118
Godstime CO, Felix OE, Augustina OJ, Christopher OE (2014) Mechanisms of antimicrobial actions of phytochemicals against enteric pathogens – A review. J Pharm Chem Biol Sci 2:77–85
Rajkumar SRJ, Nadar MSAM, Selvakumar PM (2018) Plant-derived compounds for wound healing- A review. Organic & Medicinal Chem IJ 5:555653
Simoes D, Miguel SP, Ribeiro MP, Coutinho P, Mendonca AG et al (2018) Recent advances on antimicrobial wound dressing: A review. Eur J Pharm Biopharm 127:130–141
Thangapazham RL, Sharad S, Maheshwari RK (2016) Phytochemicals in wound healing. Adv Wound Care (New Rochelle) 5:230–241
Demilew W, Adinew GM, Asrade S (2018) Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae). Evid-based Complement Altern Med 2018:1–9
Gunes OC, Albayrak AZ (2021) Antibacterial Polypeptide nisin containing cotton modified hydrogel composite wound dressings. Polym Bull 78:6409–6428
Pan Z, Ye H, Wu D (2021) Recent advances on polymeric hydrogels as wound dressings. APL Bioengineering 5:011504
Ilenghoven D, Chan CY, Kamal WSRWA, Yussof SJM, Ibrahim S (2017) A review of wound dressing practices. Clin Dermatol J 2:000133
Savencu I, Iurian S, Porfire A, Bogdan C, Tomuta I (2021) Review of advances in polymeric wound dressing films. React Funct Polym 168:105059
Dhivya S, Padma VV, Santhini E (2015) Wound dressings – a review. BioMedicine 5:24–28
Radoor S, Karayil J, Jayakumar A, Siengchin S, Parameswaranpillai J (2021) A low cost and eco-friendly membrane from polyvinyl alcohol, chitosan and honey: synthesis, characterization and antibacterial property. J Polym Res 28:82
Sharma A, Khanna S, Kaur G, Singh I (2021) Medicinal plants and their components for wound healing applications. Future J Pharm Sci 7:53
Ly HT, Nguyen MTP, Nguyen TKO, Bui TPQ, Ke X et al (2020) Phytochemical analysis and wound-healing activity of Noni (Morinda Citrifolia) leaf extract. J Herbs Spices Med Plants 26:379–393
Aslam MS, Ahmad MS, Riaz H, Raza SA, Hussain S et al (2018) Role of flavonoids as wound healing agent. Phytochemicals: IntechOpen
Kim J, Lee CM, Kim SG (2019) Phytochemical analysis and wound healing potential of ethanol extract of sea mustard and sea mustard sporophyll. Biomed Sci Lett 25:313–320
Nejjari R, Benabbes M, Amrani M, Meddah B, Bouatia M et al (2019) Phytochemical screening and wound healing activity of Telephium imperati (L.) in rats. S Afr J Bot 123:147–151
Shah A, Nik SA (2017) The role of phytochemicals in the inflammatory phase of wound healing. Int J Mol Sci 18:1068
Adeonipekun PA, Adeniyi TA, Aminu SO (2014) Investigating the phytochemicals and antimicrobial activities of shoot and root of Pycreus smithianus (Ridl.) C. B. Clarke (Family Cyperaceae). J Botany 2014:1–5
Hijji YM, Barare B, Zhang Y (2012) Lawsone (2-hydroxy-1,4-naphthoquinone) as a sensitive cyanide and acetate sensor. Sens Actuators B Chem 169:106–112
Kulkarni S, Kale V, Velankar K (2018) To study the photodynamic antimicrobial activity of Henna extract and preparation of topical gel formulation. Int J Phytopharm 7:242–252
Marzec A, Szadkowski B (2019) Improved aging stability of ethylene-norbornene composites filled with lawsone-based hybrid pigment. Polymers 11:723
Zulkifli F, Ali N, Yusof MSM, Khairul WM, Rahamathullah R et al (2017) The effect of concentration of Lawsonia inermis as a corrosion inhibitor for aluminum alloy in seawater. Adv Phys Chem 2017:1–12
Doughari JH (2006) Antimicrobial activity of Tamarindus indica Linn. Trop J Pharm Res 5:597–603
Patra AK (2012) An overview of antimicrobial properties of different classes of phytochemicals. Dietary Phytochemicals and Microbes: Springer, Dordrecht. pp. 1–32
Nwodo UU, Obiiyeke GE, Chigor VN, Okoh AI (2011) Assessment of Tamarindus indica extracts for antibacterial activity. Int J Mol Sci 12:6385–6396
Gumgumjee NM, Khedr A, Hajar AS (2012) Antimicrobial activities and chemical properties of Tamarindus indica L. leaves extract. Afr J Microbiol Res 6:6172–6181
Obulesu M, Bhattacharya S (2011) Color changes of tamarind (Tamarindus indica L.) pulp during fruit development, ripening, and storage. Int J Food Prop 14:538–549
Kaolaor A, Phunpee S, Ruktanonchai UR, Suwantong O (2019) Effects of β-cyclodextrin complexation of curcumin and quaternization of chitosan on the properties of the blend films for use as wound dressings. J Polym Res 26:43
Gajra B, Pandya SS, Vidyasagar G, Rabari H, Dedania RR et al (2012) Poly vinyl alcohol hydrogel and its pharmaceutical and biomedical applications: A review. J Int Pharm Res 4:20–26
Kader KAMAE, Hamied SFA (2002) Preparation of poly(vinyl alcohol) films with promising physical properties in comparison with commercial polyethylene film. J Appl Polym Sci 86:1219–1226
Halima NB (2016) Poly(vinyl alcohol): review of its promising applications and insights into biodegradation. RSC Adv 6:39823
Aslam M, Kalyar MA, Raza ZA (2018) Polyvinyl Alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym Eng Sci 58:2119–2132
Awada H, Daneault C (2015) Chemical modification of poly(vinyl alcohol) in water. Appl Sci 5:840–850
Mansur HS, Sadahira CM, Souza AN, Mansur AAP (2008) FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater Sci Eng C 28:539–548
Mathew S, Mathew J, Radhakrishnan EK (2019) Polyvinyl alcohol/silver nanocomposite films fabricated under the influence of solar radiation as effective antimicrobial food packaging material. J Polym Res 26:223
Mohdy HLAE (2013) Radiation synthesis of nanosilver/poly vinyl alcohol/cellulose acetate/gelatin hydrogels for wound dressing. J Polym Res 20:177
Hiremani VD, Anandalli MH, Gasti T, Dixit S, Bayannavar PK et al (2021) Dominant nature of 7-hydroxy 4-methyl coumarin dye on thermal, fluorescence and antimicrobial properties of PVA/OMS blend films. J Polym Res 28:353
Deshmukh K, Ahamed MB, Deshmukh RR, Bhagat PR, Pasha SKK et al (2016) Influence of K2CrO4 doping on the structural, optical and dielectric properties of polyvinyl alcohol/K2CrO4 composite films. Polym Plast Technol Eng 55:231–241
Choo K, Ching YC, Chuah CH, Julai S, Liou NS (2016) Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 9:644
Suganthi S, Vignesh S, Sundar JK, Raj V (2020) Fabrication of PVA polymer films with improved antibacterial activity by fine-tuning via organic acids for food packaging applications. Appl Water Sci 10:100
Wu Z, Wu J, Peng T, Li Y, Lin D et al (2017) Preparation and application of starch/polyvinyl alcohol/citric acid ternary blend antimicrobial functional food packaging films. Polymers 9:102
Sapalidis AA, Katsaros FK, Romanos GE, Kakizis NK, Kanellopoulos NK (2007) Preparation and characterization of novel poly-(vinyl alcohol)–Zostera flakes composites for packaging applications. Compos Part B 38:398–404
Kemme M, Wieland RH (2018) Quantitative assessment of antimicrobial activity of PLGA films loaded with 4-Hexylresorcinol. J Funct Biomater 9:4
Balouiri M, Sadiki M, Ibnsouda SK (2016) Methods for in vitro evaluating antimicrobial activity: A review. J Pharm Anal 6:71–79
Lourenco FR, Pinto TDJA (2011) Antibiotic microbial assay using kinetic-reading microplate system. Braz J Pharm Sci 47:573–584
Vieira DCM, Fiuza TFM, Salgado HRN (2014) Development and validation of a rapid turbidimetric assay to determine the potency of Cefuroxime Sodium in powder for injection. Pathogens 3:656–666
Li RC, Nix DE, Schentag JJ (1993) New turbidimetric assay for quantitation of viable bacterial densities. Antimicrob Agents Chemother 37:371–374
Ak Alaba, Basiru SM, Simiyu J (2011) Effect of extracting solvents on the stability and performances of dye-sensitized solar cell prepared using extract from Lawsonia Inermis. Fundamental J Modern Physics 1:261–268
Jayaprakash N, Vijaya JJ, Kaviyarasu K, Kombaiah K, Kennedy LJ et al (2017) Green synthesis of Ag nanoparticles using tamarind fruit extract for the antibacterial studies. J Photochem Photobiol B: Biol 169:178–185
Bhuiyan MAR, Islam A, Ali A, Islam MN (2017) Color and chemical constitution of natural dye henna (Lawsonia inermis L) and its application in the coloration of textiles. J Clean Prod 167:14–22
Snafi AEA (2019) A review on Lawsonia inermis: A potential medicinal plant. Int J Curr Pharm Res 11:1–13
Gomaa MM, Hugenschmidt C, Dickmann M, Hady EEA, Mohamed HFM et al (2018) Crosslinked PVA/SSA proton exchange membranes: correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys Chem Chem Phys 20:28287–28299
Kharazmi A, Faraji N, Hussin RM, Saion E, Yunus WMM et al (2015) Structural, optical, opto-thermal and thermal properties of ZnS-PVA nanofluids synthesized through a radiolytic approach. Beilstein J Nanotechnol 6:529–536
Musa MSM, Sulaiman WRW, Majid ZA, Majid ZA, Idris AK et al (2020) Henna extract as a potential sacrificial agent in reducing surfactant adsorption on kaolinite : The role of salinity. J King Saud Univ Eng Sci 32:543–547
Saadaoui S, Youssef MAB, Karoui MB, Gharbi R, Smecca E et al (2017) Performance of natural-dye-sensitized solar cells by ZnO nanorod and nanowall enhanced photoelectrodes. Beilstein J Nanotechnol 8:287–295
Muzaffar K, Dar BN, Kumar P (2017) Assessment of nutritional, physicochemical, antioxidant, structural and rheological properties of spray dried tamarind pulp powder. J Food Meas Charact 11:746–757
Chen X, Kang D, Cao L, Li J, Zhou T et al (2019) Separation and recovery of valuable metals from spent lithium ion batteries: Simultaneous recovery of Li and Co in a single step. Sep Purif Technol 210:690–697
Jayakumar S, Nandakumar T, Vadivel M, Thinaharan C, George RP et al (2020) Corrosion inhibition of mild steel in 1 M HCl using Tamarindus indica extract: electrochemical, surface and spectroscopic studies. J Adhes Sci Technol 34:713–743
Acknowledgement
The authors would like to thank Dr. R. Divakar and Dr. B. Venkataraman for their constant support and encouragement.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jayakumar, S., Philip, J. Antimicrobial property of polyvinyl alcohol films containing extracts of Lawsonia inermis and Tamarindus indica. J Polym Res 30, 108 (2023). https://doi.org/10.1007/s10965-023-03485-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03485-0