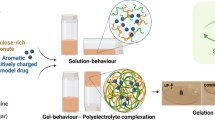
Abstract
Calcium-pectin beads are largely used for biomedical applications, however, the main drawback is their rapid disintegration in the presence of chelating and non-chelating ions from body fluids. Therefore, the principal goal of this work is to produce stable pectin beads by successive ionic and covalent cross-linking and to test their stability in simulated physiological conditions. For this purpose, native pectin was first de-esterified (DEP) to result a maximum amount of carboxylic groups, then a fraction of the DEP was oxidized with NaIO4 (OXP) to introduce aldehyde groups susceptible to covalent cross-linking. Finally, the de-methylated and de-methylated/oxidized pectin were mixed and transformed into beads by double cross-linking: ionic with calcium ions and covalent with adipic acid dihydrazide (ADH). The gelling properties, sphericity and shape as well as the morphology and the stability of the beads in different media were investigated. Finally, beads were tested for their capacity to encapsulate and release drug molecule. Therefore, microcapsules were loaded with FITC-dextran, a standard high molecular weight model drug molecule, with high encapsulation efficiency. A remarkable delay in FITC-dextran release was observed for DEP/OXP beads compared to DEP particles. The transport mechanism of solvent and FITC-dextran in/from the DEP/OXP beads was determined as a Fickian diffusion-driven. The viability tests proved that both simple and double cross-linked microcapsules are cytocompatible for the HEK-293 cells at pectin concentrations up to 5.5 mg/mL.










Similar content being viewed by others
Data availability
The data required will be available on demand.
References
Celus M, Kyomugasho C, Van Loey AM, Grauwet T, Hendrickx ME (2018) Influence of pectin structural properties on interactions with divalent cations and its associated functionalities. Compr Rev Food Sci Food Saf 17:1576–1594. https://doi.org/10.1111/1541-4337.12394
Chen D, Chang L, Zhou Z, Bo Y, Wang Y, He Y, Qin J (2021) Pectin-based self-healing hydrogel with NaHCO3 degradability for drug loading and release. J Polym Res 28:59. https://doi.org/10.1007/s10965-021-02430-3
Sande SA (2005) Pectin-based oral drug delivery to the colon. Expert Opin Drug Deliv 2:441–450. https://doi.org/10.1517/17425247.2.3.441
Schiewer S, Patil SB (2008) Pectin-rich fruit wastes as biosorbents for heavy metal removal: Equilibrium and kinetics. Biores Technol 9:1896–1903. https://doi.org/10.1016/j.biortech.2007.03.060
Eliaz I, Hotchkiss AT, Fishman ML, Rode D (2006) The effect of modified citrus pectin on urinary excretion of toxic elements. Phytoter Res 20:859–864. https://doi.org/10.1002/ptr.1953
Gant GT, Morris ER, Rees DA, Smith PJC, Thom D (1973) Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett 32:195–198. https://doi.org/10.1016/0014-5793(73)80770-7
Braccini I, Pérez S (2001) Molecular basis of Ca2+-induced gelation in alginates and pectins: The egg-box model revisited. Biomacromol 2:1089–1096. https://doi.org/10.1021/bm010008g
Assifaoui A, Lerbret A, Uyen HTD, Neiers F, Chambin O, Loupiac C, Cousin F (2015) Structural behaviour differences in low methoxy pectin solutions in the presence of divalent cations (Ca2+ and Zn2+): a process driven by the binding mechanism of the cation with the galacturonate unit. Soft Matter 11:551–560. https://doi.org/10.1039/C4SM01839G
Jung J, Arnold RD, Wicker L (2013) Pectin and charge modified pectin hydrogel beads as a colon-targeted drug delivery carrier. Colloid Surface B 104:116–121. https://doi.org/10.1016/j.colsurfb.2012.11.042
Babaladimath G, Badalamoole V (2018) Pectin-graft-poly(2-acrylamido-2-methyl-1-propane sulfonic acid) silver nanocomposite hydrogel beads: evaluation as matrix material for sustained release formulations of ketoprofen and antibacterial assay. J Polym Res 25:202. https://doi.org/10.1007/s10965-018-1592-5
Jantrawut P, Chambin O, Ruksiriwanich W (2015) Scavenging activity of rutin encapsulated in low methoxyl pectin beads. Cell Chem Technol 49:51–54
Atara SA, Soniwala M (2018) Formulation and evaluation of pectin-calcium chloride beads of azathioprine for colon targeted drug delivery system. Int J Pharm Pharm Sci 10:172–177. https://doi.org/10.22159/ijpps.2018v10i1.23175
Berger R, Ruhlemann I (1988) Stable ionotropic gel for immobilization using high molecular weight pectic acid. Acta Biotechnol 8:401–405. https://doi.org/10.1002/abio.370080503
Li M, Jin Y, Wang Y, Meng L, Zhang N, Sun Y, Hao J, Fu Q (2019) Preparation of Bifidobacterium breve encapsulated in low mehoxyl pectin beds and its effect on yogurt quality. J Dairy Sci 102:1–12. https://doi.org/10.3168/jds.2018-15597
Chang KLB, Lin J (2000) Swelling behavior and the release of protein from chitosan-pectin composite particles. Carbohydr Polym 43:163–169. https://doi.org/10.1016/S0144-8617(00)00145-4
Kim TH, Park YH, Kim KJ, Cho CS (2003) Release of albumin from chitosan-coated pectin beads in vitro. Int J Pharm 250:371–383. https://doi.org/10.1016/S0378-5173(02)00553-7
Bourgeois S, Laham A, Besnard M, Andremont A, Fattal E (2005) In vitro and in-vivo evaluation of pectin beads for the colon delivery of β-lactamases. J Drug Target 13:277–284. https://doi.org/10.1080/10611860500206583
de Souza JRR, de Carvalho JIX, Trevisan MTS, de Paula RCM, Ricardo NMPS, Feitosa JPA (2009) Chitosan-coated pectin beads: Characterization and in vitro release of mangiferin. Food Hydrocoll 23:2278–2286. https://doi.org/10.1016/j.foodhyd.2009.06.004
Cabrera JC, Cambier P, van Cutsem P (2011) Drug encapsulation in pectin hydrogel beads- a systematic study of simulated digestion media. Int J Pharm Pharm Sci 3:292–299
Liu LS, Fishman ML, Hicks KB, Kende M, Ruthel G (2006) Pectin/zein beads for potential colon-specific drug delivery: synthesys and in vitro evaluation. Drug Deliv 13:417–423. https://doi.org/10.1080/10717540500394935
Das S, Ng K (2010) Impact of glutaraldehyde on in vivo colon-specific release of resveratrol from biodegradable pectin-based formulation. J Pharm Sci 99:4903–4916. https://doi.org/10.1002/jps.22212
Das S, Ng K, Ho PC (2011) Design of a pectin-based microparticle formulation using zinc ions as the cross-linking agent and glutaraldehyde as the hardening agent for colonic-specific delivery of resveratrol: In vitro and in vivo evaluations. J Drug Traget 19:446–457. https://doi.org/10.3109/1061186X.2010.504272
Vityazev FV, Khramova DS, Saveliev NY, Ipatova EA, Burkov AA, Beloserov VS, Belyi VA, Kononov LO, Martinson EA, Litvinets SG, Markov PA, Popov SV (2020) Pectin–glycerol gel beads: preparation, characterization and swelling behaviour. Carbohydr Polym 238(15). https://doi.org/10.1016/j.carbpol.2020.116166
Vityazev FV, Fedyuneva MI, Golovchenko VV, Patova OA, Ipatova EU, Durnev EA, Martinson EA, Litvinets SG (2017) Pectin-silica gels as matrices for controlled drug release in gastrointestinal tract. Carbohydr Polym 157:9–20. https://doi.org/10.1016/j.carbpol.2016.09.048
Kristiansen KA, Potthast A, Christensen BE (2010) Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr Res 345:1264–1271. https://doi.org/10.1016/j.carres.2010.02.011
Gupta B, Tummalapalli M, Deopura BL, Alam MS (2013) Functioanlization of pectin by periodate oxidation. Carbohydr Polym 98:1160–1165. https://doi.org/10.1016/j.carbpol.2013.06.069
Chetouani A, Elkolli M, Bounekhel M, Benachour D (2014) Synthesis and properties of novel hydrogels from oxidized pectin crosslinked gelatin for biomedical applications. Polym Bull 71:2303–2316. https://doi.org/10.1007/s00289-014-1189-z
Munarin F, Petrini P, Tanzi MC, Barbosa MA, Granja PL (2012) Biofunctional chemically modified pectin for cell delivery. Soft Matter 8:4731–4739. https://doi.org/10.1039/C2SM07260B
Chetouani A, Follain N, Marais S, Rihouey C, Elkolli M, Bounekhel M, Benachour D, LeCerf D (2017) Physicochemical properties and biological activities of novel blend films using oxidized pectin/chitosan. Int J Biol Macromol 97:348–356. https://doi.org/10.1016/j.ijbiomac.2017.01.018
Chen S, Cui S, Zhang H, Pei X, Hu J, Zhou YZ, Liu Y (2018) Crosslinked pectin nanofibers with enhanced cell adhesion. Biomacromol 19:490–498. https://doi.org/10.1021/acs.biomac.7b01605
Tummalapali M, Gupta B (2015) A UV-Vis spectrophotometric method for the estimation of aldehyde groups in periodate-oxidized polysaccharides using 2,4-dinitrophenyl hydrazine. J Carbohydr Chem 34:338–348. https://doi.org/10.1080/07328303.2015.1068793
Morris GA, Foster TJ, Harding SE (2002) A hydrodynamic study of the depolymerisation of a high methoxy pectin at elevated temperatures. Carbohydr Polym 48:361–367. https://doi.org/10.1016/S0144-8617(01)00270-3
Morris GA, Castile J, Smith A, Adams GG, Harding SE (2010) The effect of different storage temperatures on the physical properties of pectin solutions and gels. Polym Degrad Stabil 95:2670–3267. https://doi.org/10.1016/j.polymdegradstab.2010.07.013
Bruneel D, Schacht E (1993) Chemical modification of pullulan: 1. Periodate oxidation Polymer 34:2628–2632. https://doi.org/10.1016/0032-3861(93)90600-F
Ritger PL, Peppas NA (1987) A simple equation for description of solute release II. Fickian and anomalous release from swellable device. J Controlled Release 5:37–42. https://doi.org/10.1016/0168-3659(87)90035-6
Bruschi ML (2015) Mathematical models of drug release In: Bruschi ML (ed) Strategies to modify the drug release from pharmaceutical systems, 1st edn. Woodhead Publishing, Cambridge, pp 63–86. https://doi.org/10.1016/C2014-0-02342-8
Padival RA, Ranganna S, Manjrekar SP (1979) Low methoxyl pectin from lime peel. J Fd Technol 14:333–343. https://doi.org/10.1111/j.1365-2621.1979.tb00878.x
Rosenbohm C, Lundt I, Christensen TMIE, Young NWG (2003) Chemically methylated and reduced pectins: preparation, characterisation by 1H NMR spectroscopy, enzymatic degradation, and gelling properties. Carbohydr Res 338:637–649. https://doi.org/10.1016/S0008-6215(02)00440-8
Filippov MP, Kohn R (1975) Determination of the esterification degree of carboxyl groups of pectin with methanol by means of infrared spectroscopy. Chem Zvesti 29(1):88–91
Fellah A, Anjukandi P, Waterland MR, Williams MAK (2009) Determining the degree of methylesterification of pectin by ATR/FT-IR: Methodology optimisation and comparison with theoretical calculations. Carbohydr Polym 78:847–853. https://doi.org/10.1016/j.carbpol.2009.07.003
Synystsya A, Copikova J, Matejka P, Machovic V (2003) Fourrier transform Raman and infrared spectroscopy of pectins. Carbohydr Polym 54:97–106. https://doi.org/10.1016/S0144-8617(03)00158-9
Baum A, Dominiak M, Vidal-Melgosa S, Willats WGT, Søndergaard KM, Hansen PW et al (2017) Prediction of pectin yield and quality by FTIR and carbohydrate microarray analysis. Food Bioproc Tech 10:143–154. https://doi.org/10.1007/s11947-016-1802-2
Huamani-Palomino RG, Cordova BM, Pichilingue LER, Venancio T, Valderrama AC (2021) Functionalization of an Alginate-based material by oxidation and reductive amination. Polymers 13:255–269. https://doi.org/10.3390/polym13020255
Ayarza J, Coello Y, Nakamatsu J (2017) SEM-EDS study of ionically crosslinked alginate and alginic acid bead formation. Int J Polym Anal Charact 22:1–10. https://doi.org/10.1080/1023666X.2016.1219834
Thu B, Gåserød O, Paus D, Mikkelsen A, Skjak-Braek G, Toffanin R, Vittur F, Rizzo R (2000) Inhomogeneous alginate gel spheres: an assessment of the polymer gradients by synchrotron radiation-induced X-ray emission, magnetic resonance microimaging, and mathematical modeling. Biopolymers 53:60–77. https://doi.org/10.1002/(SICI)1097-0282(200001)53:1%3c60::AID-BIP6%3e3.0.CO;2-F
Pretsch E, Bühlmann P, Badertscher M (2010) Spektroskopische Daten zur Strukturaufklarung organischer Verbindungen. Springer, Heidelberg
Cinarli A, Gürbüz D, Tavman A, Birteksöz AS (2011) Synthesis, spectral characterizations and antimicrobial activity of some Schiff bases of 4-chloro-2-aminophenol. Bull Chem Soc Ethiop 25:407–417. https://doi.org/10.4314/bcse.v25i3.68593
Pawar A, Gadhe A, Venkatachalam P, Sher P, Mohadik K (2008) Effect of core and surface cross-linking on the entrapment of metronidazole in pectin beads. Acta Pharm 58:78–85. https://doi.org/10.2478/v10007-007-0046-0
Rebitski EP, Darder M, Carraro R, Aranda P, Ruiz-Hitzky E (2020) Chitosan and pectin core–shell beads encapsulating metformin–clay intercalation compounds for controlled delivery. New J Chem 44:10102–10110. https://doi.org/10.1039/C9NJ06433H
Remunán-López C, Bodmeier R (1997) Mechanical, water uptake and permeability properties of crosslinked chitosan glutamate and alginate films. J Control Rel 44:215–225. https://doi.org/10.1016/S0168-3659(96)01525-8
Agnihotri SA, Jawalkar SS, Aminabhavi TM (2006) Controlled release of cephalexin through gellan gum beads: effect of formulation parameters on entrapment efficiency, size and drug release. Eur J Pharm Biopharm 63:249–261. https://doi.org/10.1016/j.ejpb.2005.12.008
Siepmann J, Siepmann F (2008) Mathematical modeling of drug delivery. Int J Pharm 364:328–343. https://doi.org/10.1016/j.ijpharm.2008.09.004
Funding
This work was supported by a grant of the Romanian Ministry of Research and Innovation, CCDI-UEFISCDI, project number PN-III-P1-1.2.-PCCDI-2017–0697/contract nr. 13PCCDI/2018 within PNCDI III (INTERA) and by the European Social Fund for Regional Development, Competitiveness Operational Programme Axis 1 – Project “Petru Poni” Institute of Macromolecular Chemistry-Interdisciplinary Pol for Smart Specialization through Research and Innovation and Technology Transfer in Bio(nano)polymeric Materials and (Eco)Technology”, InoMatPol (ID P_36_570, Contract 142/10.10.2016, cod MySMIS: 107464).
Author information
Authors and Affiliations
Contributions
Conceptualization: Marieta Constantin and Gheorghe Fundueanu; Methodology: Irina Popescu and Marieta Constantin; Investigation: Mihail Lupei, Irina Popescu, Geanina Voicu, and Anca Irina Prisacaru; Writing – original draft preparation: Irina Popescu and Marieta Constantin; Writing—review and editing: Gheorghe Fundueanu and Manuela Calin; Supervision: Gheorghe Fundueanu.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflicts of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Popescu, I., Lupei, M., Constantin, M. et al. Double cross-linked pectin beads stable in physiological environment as potential support for biomedical applications. J Polym Res 28, 424 (2021). https://doi.org/10.1007/s10965-021-02779-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-021-02779-5