Abstract
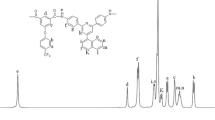
A new diaroyl chloride monomer, 5-(4-benzoyl-2,3,5,6-tetrafluorophenoxy)isophthaloyl dichloride (BTFPIPC), was prepared in a three-step synthesis. Six novel aromatic polyamides containing 4-benzoyl-2,3,5,6-tetrafluorophenoxy pendant groups were synthesized by low temperature polycondensation of BTFPIPC with six aromatic diamines in N,N-dimethylacetamide (DMAc). All these new polymers are amorphous and readily soluble in various dipolar solvents such as DMAc, N-methyl-2-pyrrolidinone (NMP), N,N-dimethylformamide (DMF), and dimethyl sulfoxide (DMSO) at room temperature. These polymers showed glass transition temperatures between 212 and 243 °C and 5% weight loss temperatures ranging from 439 °C to 456 °C. These polyamides could be cast into transparent, flexible and strong films from DMAc solution with tensile strengths of 73.5–85.4 MPa, tensile moduli of 2.06–2.72 GPa, and elongations at break of 6.4–9.3%. These new polyamide films exhibited low dielectric constants of 3.26–3.57 (1 MHz), lower water uptakes in the range of 1.27–2.28%, and excellent transparency with an ultraviolet-visible absorption cut-off wavelength in the 326–373 nm range. Primary characterization of these new polyamides shows that they might serve as new candidates for processable high-performance polymeric materials.







Similar content being viewed by others
References
Oishi Y, Kakimoto M, Imai Y (1988). Macromolecules 21:547–550
Yang HH (1989) Aromatic high-strength fibers. Wiley, New York, pp 66–289
Liou GS, Hsiao SH (2002). J Polym Sci Part A Polym Chem 40:2564–2574
Wu SC, Shu CF (2003). J Polym Sci Part A Polym Chem 41:1160–1166
Liaw DJ, Hsu PN, Chen WH, Lin SL (2002). Macromolecules 35:4669–4675
Garcia JM, Garcia FC (2010) Serna F, de laPena JL. Prog Polym Sci 35:623–686
Marchildon K (2011). Macromol React Eng 5:22–54
Hsiao SH, Huang PC (1997). Macromol Chem Phys 198:4001–4009
Yang CP, Lin JH (1994). J Polym Sci Part A Polym Chem 32:423–433
Ghaemy M, Barghamadi M (2008). J Appl Polym Sci 110:1730–1738
Chen J-C, Rajendran K, Huang S-W, Chang H-W (2011). J Polym Res 18:1693–1703
Zeng K, Hong HB, Zhou SH, Wu DM, Miao PK, Huang ZF, Yang G (2009). Polymer 50:5002–5006
Yen HJ, Liou GS (2008). J Polym Sci Part A Polym Chem 46:7354–7368
Behniafar H, Khosravi-borna S (2009). Polym Int 58:1299–1307
Espeso JF, Lozano AE, de la Campa JG, Gaecia-Yoldi I, de Abajo J (2010). J Polym Sci Part A Polym Chem 48:1743–1751
Hsiao S-H, Wang H-M, Chou J-S, Guo W, Tsai T-H (2012). J Polym Res 19:9902
Dinari M, Haghighi A (2017). J Polym Res 24:29
Hsiao SH, Yang CP, Lin WL (1999). Macromol Chem Phys 200:1428–1433
Maji S, Sen SK, Dasgupta B, Chatterjee S, Banerjee S (2009). Polym Adv Technol 20:384–392
Sheng SR, Ma CX, Jiang JW, Huang ZZ, Song CS (2010). J Appl Polym Sci 116:1650–1659
Hsiao SH, Lin KH (2004). Polymer 45:7877–7885
Pal SS, Patil PS, Salunkhe MM, Maldar NN, Wadgaonkar PP (2009). Eur Polym J 45:953–959
Damaceanu MD, Rusu RD, Nicolescu A, Bruma M, Rusanov AL (2011). Polym Int 60:1248–1258
Agata Y, Kobayashi M, Kimura H, Takeishi M (2005). Polym Int 54:260–266
Liang QZ, Liu PT, Liu C, Jian XG, Hong DY, Li Y (2005). Polymer 46:6258–6265
Liaw DJ, Chang FC, Leung MK, Chou MY, Muellen K (2005). Macromolecules 38:4024–4029
Liaw DJ, Huang CC, Chen WH (2006). Polymer 47:2337–2348
Hsiao S-H, Wang H-M, Chang P-C, Kung Y-R (2013). Lee T-M 20:154
Maier G (2001). Prog Polym Sci 26:3–65
Dhara MG, Banerjee S (2010). Prog Polym Sci 35:1022–1077
Hsiao SH, Yang CP, Tsai CY, Liou GS (2004). Eur Polym J 40:1081–1094
Ge ZY, Yang SY, Tao ZQ, Liu JG, Fan L (2004). Polymer 45:3627–3635
Liaw DJ, Chen WH, Hu CK, Lee KR, Lai JY (2007). Polymer 48:6571–6580
Sheng SR, Pei XL, Liu XL, Song CS (2009). Eur Polym J 45:230–236
Behniafar H, Sedaghatdoost M (2011). J Fluor Chem 132:276–284
Liu XL, Wu D, Sun R, Yu LM, Jiang JW, Sheng SR (2013). J Fluor Chem 154:16–32
Cai M, Zhu M, Wang P, Song C (2010). Polymer 51:1293–1300
Cai M, Chen M, Yu Y, Song C (2013). Polym Adv Technol 24:466–472
Zhu X, Yan T, Xu Q, Cai M (2016). J Polym Res 23:24
Zou F, Wen H, Yan T, Cai M (2016). J Polym Res 23:225
Houghan G, Tesoro G, Viehbeck A (1996). Macromolecules 29:3453–3456
Acknowledgments
The authors thank the National Natural Science Foundation of China (Project 21664008) and the Natural Science Foundation of Jiangxi Province of China (Project 20132BAB203015) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tang, H., Huang, B., Xie, X. et al. Synthesis and properties of novel soluble fluorinated aromatic polyamides containing 4-benzoyl-2,3,5,6-tetrafluorophenoxy pendant groups. J Polym Res 25, 51 (2018). https://doi.org/10.1007/s10965-018-1454-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1454-1