Abstract
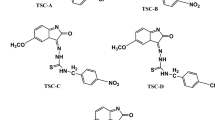
The electronic structures, solvatochromism, and LSER (Linear Solvation Energy Relationship) properties of thiosemicarbazone derivatives having five different π-conjuged systems have been investigated. UV–Vis absorption spectra of TSC (Thiosemicarbazone) derivatives have been measured in 18 solvent media with different polarities. The global electronic transition in these compounds is described as π−π* electronic transition resulting from the resonance in the thiosemicarbazone group. The other lower wavelength maxima are denoted to the π−π* electronic transition in the indole ring and the n−π* electronic transition in the carboxyl group. To determine as analytical solvent–solute interactions of global electronic transitions has been studied with using LSERs with using Kamlet–Taft and Catalán parameters. The solvatochromic models between electronic transition wavenumbers with Reichardt parameter, and Marcus solvent function have been generated. Optoelectronic properties have been investigated using the Moss, Ravindra, Herve-Vándamme, Kumar, Singh, and Reddy relationships. According to Eg (molecular energy gap) values, the investigated compounds can be considered organic semiconductor materials.
Graphical Abstract







Similar content being viewed by others
References
Hussain, M., Jawaria, R., Shafiq, Z., Abbas, G., Naseer, M.M.: Ferrocene-based thiosemicarbazones: solvent effect on thiol-thione tautomerism and conformational polymorphism. J. Organomet. Chem. 846, 121–128 (2017). https://doi.org/10.1016/j.jorganchem.2017.05.005
Bingül, M.: Synthesis of indole-3-carboxyaldehyde thiosemicarbazone derivatives and investigation of antioxidant and anticholinesterase properties. Afyon Kocatepe Üniversitesi Fen ve Mühendislik Bilimleri Dergisi 19, 317–327 (2019). https://doi.org/10.35414/akufemubid.542712
Tom, L., Aiswarya, N., Sreejith, S.S., Kurup, M.P.: Self-organized three dimensional architectures based on non-covalent interactions in square planar Cu (II) thiosemicarbazone: solvent mediated crystallization and EPR based correlation study. Inorg. Chim. Acta 473, 223–235 (2018). https://doi.org/10.1016/j.ica.2018.01.005
İlhan-Ceylan, B., Bolukbasi, O., Yilmaz, A., Kaya, K., Kurt, Y., Ülküseven, B.: Synthesis, spectroscopic characterization and quantum chemical studies of a dioxomolybdenum (VI) complex with an N S-substituted pyridoxal thiosemicarbazone. Polyhedron 193, 114884 (2021). https://doi.org/10.1016/j.poly.2020.114884
Gülseven, Y., Taşal, E., Sıdır, İ, Güngör, T., Berber, H., Öğretir, C.: Solvatochromic effect studies on the absorption spectra of 4-((2-ethylphenyl)diazenyl)benzene-1,3-diol and 2-((2-ethylphenyl)diazenyl)benzene-1,3,5-triol molecules. Int. J. Hydrog. Energy 34(12), 5255–5259 (2009). https://doi.org/10.1016/j.ijhydene.2008.10.070
Gülseven Sıdır, Y., Sıdır, İ, Taşal, E., Ermiş, E.: Studies on the electronic absorption spectra of some monoazo derivatives. Spectrochim. Acta Part A 78, 640–647 (2011). https://doi.org/10.1016/j.saa.2010.11.040
Gülseven Sıdır, Y., Sıdır, İ, Berber, H., Taşal, E.: UV-spectral changes for some azo compounds in the presence of different solvents. J. Mol. Liq. 162, 148–154 (2011). https://doi.org/10.1016/j.molliq.2011.07.002
Sıdır, İ, Gülseven Sıdır, Y., Berber, H., Taşal, E.: A study on solvatochromism of some monoazo dye derivatives. J. Mol. Liq. 178, 127–136 (2013). https://doi.org/10.1016/j.molliq.2012.11.011
Gülseven Sıdır, Y., Sıdır, İ, Berber, H., Türkoğlu, G.: Solvatochromic behavior and electronic structure of some symmetric 2-aminophenol Schiff base derivatives. J. Mol. Liq. (2014). https://doi.org/10.1016/j.molliq.2014.08.018
Gülseven Sıdır, Y., Berber, H., Sıdır, İ: The solvatochromism, ground&excited state electric dipole moments and molecular electronic properties of ((4-phenoxybenzyliden)amino)phenol compounds. J. Solution Chem. 48, 775–806 (2019). https://doi.org/10.1007/s10953-019-00885-z
Gülseven Sıdır, Y.: The solvatochromism, electronic structure, electric dipole moments and DFT calculations of benzoic acid liquid crystals. Liq. Crystals 47(10), 1435–1451 (2020). https://doi.org/10.1080/02678292.2020.1733685
Sıdır, İ, Gülseven Sıdır, Y.: Investigation on the interactions of E-4 methoxycinnamic acid with solvent: Solvatochromism, electric dipole moment and pH effect. J. Mol. Liq. 249, 1161–1171 (2018). https://doi.org/10.1016/j.molliq.2017.11.136
Kandemirli, F., Choudhary, M.I., Siddiq, S., Saracoglu, M., Sayiner, H., Arslan, T., Erbay, A., Köksoy, B.: Quantum chemical calculations for some ısatin thiosemicarbazones. Quantum Chem. Mol. Innovat. 3, 25–58 (2012)
Ganim, M.A., Baloglu, M.C., Aygun, A., Altunoglu, Y.C., Sayiner, H.S., Kandemirli, F., Sen, F.: Analysis of DNA protection, interaction and antimicrobial activity of isatin derivatives. Int J. Biol. Macromol. 122, 1271–1278 (2019). https://doi.org/10.1016/j.ijbiomac.2018.09.084
Kandemirli, F., Akkaya, Y., Vurdu, C.D.: Synthesis and quantum chemical calculations of 4-(2-fluorophenyl)-1-(2-oxoindolin-3-ylidene)thiosemicarbazone and its zinc(II) complex. Asian J. Chem. (2013). https://doi.org/10.14233/ajchem.2013.15221
Kandemirli, F., Arslan, T., Koksoy, B., Yilmaz, M.: Synthesis, characterization and theoretical calculations of 5-methoxyisatin-3-thiosemicarbazone derivatives. J. Chem. Soc. Pak. 31(3), 498–504 (2009)
Kamlet, M.J., Abboud, J.L.M., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships 23: A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. (1983). https://doi.org/10.1021/jo00165a018
Reichardt, C.: Solvents and Solvent Effects in Organic Chemistry. VCH, New York (2008)
Reichardt, C., Welton, T.: Solvents and Solvent Effects in Organic Chemistry. Wiley, New York (2011)
Kamlet, M.J., Taft, R.W.: The solvatochromic comparison method 2: the alpha-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 98, 2886 (1976). https://doi.org/10.1021/ja00426a036
Abboud, J.L.M., Kamlet, M.J., Taft, R.W.: An examination of linear solvation energy relationships. Prog. Phy. Org. Chem. 13, 485 (1981). https://doi.org/10.1002/9780470171929.ch6
Koppel, I.A., Palm, V.M.: The Influence of the solvent on organic reactivity. In: Chapman, N.B., Shorter, J.S. (eds.) Advances in Linear Free Energy Relationships. Plenum Press, London (1972)
Valeur, B.: Molecular Fluorescence-Principles and Applications. Wiley, Weinheim (2002)
Catalán, J.: Toward a generalized treatment of the solvent effect based on four empirical scales: dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J. Phys. Chem. B 113(17), 5951–5960 (2009). https://doi.org/10.1021/jp8095727
Marcus, R.A.: Free energy of nonequilibrium polarization systems. III. statistical mechanics of homogeneous and electrode systems. J. Chem. Phys. 39, 1734 (1963). https://doi.org/10.1063/1.1734522
Marcus, R.A.: On the theory of shifts and broadening of electronic spectra of polar solutes in polar media. J. Chem. Phys. 43, 1261 (1965). https://doi.org/10.1063/1.1696913
Reichardt, C.: Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 24, 2319–2358 (1994). https://doi.org/10.1021/cr00032a005
Tauc, J., Menth, A.: States in the gap. J. Non-Crystal. Solid. 8–10, 569–585 (1972). https://doi.org/10.1016/0022-3093(72)90194-9
Tauc, J.: Amorphous and Liquid Semiconductors. Plenum Press, New York (2000)
El-Asmy, A.A., Shaibi, Y.M., Shedaiwa, I.M., Khattab, M.A.: Coordination compounds of some thiosemicarbazone derivatives: their preparation, characterization and electrical properties. Synth. React. Inorg. Met.-Org. Chem. (1990). https://doi.org/10.1080/00945719008048148
Moss, T.S.: Relations between the refractive index and energy gap of semiconductors. Phys. Stat. Sol. B 131, 415 (1985). https://doi.org/10.1002/pssb.2221310202
Moss, T.S.: A Relationship between the refractive index and the infra-red threshold of sensitivity for photoconductors. Proc. Phys. Soc. B (1950). https://doi.org/10.1088/0370-1301/63/3/302
Ravindra, N.M., Auluck, S., Srivastava, V.K.: On the penn gap in semiconductors. Phys. Stat. Sol. B 93(2), K155–K160 (1979). https://doi.org/10.1002/pssb.2220930257
Herve, P., Vandamme, L.K.J.: General relation between refractive index and energy gap in semiconductors. Infrared Phys. Technol. 35(4), 609–615 (1994). https://doi.org/10.1016/1350-4495(94)90026-4
Kumar, V., Singh, J.K.: Model for calculating the refractive index of different materials. Indian J. Pure Appl. Phys. 48(8), 571–574 (2010)
Reddy, R., Anjaneyulu, S.: Analysis of the Moss and Ravindra relations. Phys. Stat. Sol. B (1992). https://doi.org/10.1002/pssb.2221740238
Akinchan, N.T., Akinchan, R.: Synthesis and spectroscopic studies on platinum (II) complexes of thiosemicarbazone derivatives of p-anisaldehyde, p-tolualdehyde and p-vanilin. Indian J. Chem. 41A, 1152–1156 (2002)
Hansch, C., Leo, A., Taft, R.W.: A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991). https://doi.org/10.1021/cr00002a004
Chapman, N.B., Shorter, J. (eds.): Correlation Analysis in Chemistry : Recent Advances. Plenum Press, New York (2012)
Aaron, J.J., Tive, A., Villiers, C., Parklanyi, C., Bouin, D.: Electronic absorption and fluorescence spectra of indole derivatives: quantatie treatment of the substituent effects and a theoretical study. Croatia Chem. Acta 56(2), 157–168 (1983)
Acknowledgements
The authors thanks Bitlis Eren University for their support to the BEBAP2013.04 and BEBAP2014.05 projects.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kara, Y.E., Sıdır, Y.G., Sıdır, İ. et al. Solvatochromism and Optoelectronic Properties of Thiosemicarbazone Derivatives Having π-Conjugated Systems. J Solution Chem 52, 570–587 (2023). https://doi.org/10.1007/s10953-023-01248-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-023-01248-5