Abstract
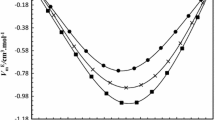
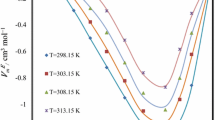
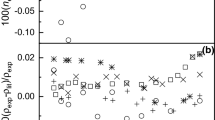
Densities and refractive indices are investigated for four binary mixture systems of dimethylvinylethoxysilane with n-heptane, n-octane, n-nonane and n-decane at different temperatures (298.15, 303.15, 308.15, 313.15 and 318.15 K) and atmospheric pressure. From these experimental data, the excess molar volume, isobaric coefficient of thermal expansion, deviation in refractive index, molar refraction, and deviation in the molar refraction have been calculated. The computed quantities have been fitted with the Redlich–Kister equation to derive the coefficients and estimate the standard deviations. The values of the partial excess volume at infinite dilution were calculated using the coefficients of the Redlich–Kister smoothing equation. The effects of composition and temperature on these properties are discussed.







Similar content being viewed by others
References
Dong, H., Yue, Y., Wu, C., Lai, G.Q.: Excess molar volumes of 2,4,6,8-tetramethylcyclotetrasiloxane with benzene, toluene, and xylene at T = (288.15, 298.15, and 308.15) K. J. Chem. Eng. Data 57, 1050–1056 (2012)
Zhang, Y.D., Dong, H., Yue, Y., Wu, C.: Effect of temperature and composition on the density, refractive index, and excess quantities of binary mixtures of 2,4,6,8-tetramethyl-2,4,6,8-tetraethenylcyclotetrasiloxane with aromatic hydrocarbons. J. Chem. Thermodyn. 57, 114–130 (2013)
Yu, L.J., Dong, H., Wu, C., Zhang, Y.D.: The density, refractive index, and thermodynamic behaviour of binary mixtures of 1,3-diethenyl-1,1,3,3-tetramethyldisiloxane with aromatic hydrocarbons. J. Chem. Thermodyn. 72, 139–151 (2014)
Zhang, Y.D., Dong, H., Wu, C., Yu, L.J.: Thermophysical properties of binary mixtures of triethoxysilane, methyltriethoxysilane, vinyltriethoxysilane and 3-mercaptopropyltriethoxysilane with ethylbenzene at various temperatures. J. Chem. Thermodyn. 76, 45–55 (2014)
Yu, L.J., Hu, Y.Q., Dong, H., Wu, C.: The physicochemical properties of 1,3,5-trimethyl-1,3,5-tris(3,3,3-trifluoropropyl)cyclotrisiloxane with various aromatic hydrocarbons at T = (308.15 to 323.15) K. J. Chem. Thermodyn. 96, 117–126 (2016)
Brown, I., Ewald, A.H.: Liquid–vapour equilibria. II. The system benzene–n-heptane. Aust. J. Chem. 4, 198–212 (1951)
Pomerantz, P.: Synthesis and physical properties of n-heptane and 2,2,4-trimethylpentane. J. Res. Nat. Bur. Stand. 48, 76–81 (1952)
Fang, S., Fu, Y.Y., Wang, Q., Zhang, G.Y.: Mixing properties of tris(2-ethylhexyl) phosphate with alkanes at different temperatures and data treatment using several correlation equations based on Eyring’s absolute reaction theory. J. Mol. Liq. 154, 111–116 (2010)
Fang, S., Zhao, C.X., He, C.H.: Densities and viscosities of binary mixtures of tri-n-butyl phosphate + cyclohexane, + n-heptane at T = (288.15, 293.15, 298.15, 303.15, and 308.15) K. J. Chem. Eng. Data 53, 2244–2246 (2008)
Yang, X., Xing, Y., Li, D., Guo, Y.S., Fang, W.J.: Volumetric and viscous properties at several temperatures for binary mixtures of n-methylpiperazine with methylcyclohexane or n-heptane. J. Chem. Eng. Data 55, 2914–2916 (2010)
Landaverde-Cortes, D.C., Estrada-Baltazar, A., Iglesias-Silva, G.A.: Densities and viscosities of MTBE + heptane or octane at p = 0.1 MPa from (273.15 to 363.15) K. J. Chem. Eng. Data 52, 1226–1232 (2007)
Lei, Y.T., Chen, Z.Y., An, X.Q., Huang, M.J., Shen, W.G.: Measurements of density and heat capacity for binary mixtures {x benzonitrile + (1 − x) (octane or nonane)}. J. Chem. Eng. Data 55, 4154–4161 (2010)
González, E.J., Calvar, N., Gómez, E., Domínguez, Á.: Separation of benzene from alkanes using 1-ethyl-3-methylpyridinium ethylsulfate ionic liquid at several temperatures and atmospheric pressure: effect of the size of the aliphatic hydrocarbons. J. Chem. Thermodyn. 42, 104–109 (2010)
Rathnam, M.V., Mankumare, S., Kumar, M.S.S.: Density, viscosity, and speed of sound of (methyl benzoate + cyclohexane), (methyl benzoate + n-hexane), (methyl benzoate + heptane), and (methyl benzoate + octane) at temperatures of (303.15, 308.15, and 313.15) K. J. Chem. Eng. Data 55, 1354–1358 (2010)
Balán, J., Morávková, L., Linek, J.: Excess molar volumes of the (cyclohexane + pentane, or hexane, or heptane, or octane, or nonane) systems at the temperature 298.15 K. Chem. Pap. 61, 497–501 (2007)
Lago, A., Rivas, M.A., Legido, J., Iglesias, T.P.: Study of static permittivity and density of the systems (n-nonane + monoglyme or diglyme) at various temperatures. J. Chem. Thermodyn. 41, 257–264 (2009)
Landaverde-Cortes, D.C., Iglesias-Silva, G.A.: Densities and viscosities of MTBE + nonane or decane at p = 0.1 MPa from (273.15 to 363.15) K. J. Chem. Eng. Data 53, 288–292 (2008)
de Cominges, B.E., Pineiro, M.M., Mascato, E., Mosteiro, L., Lglesias, T.P., Legido, J.L.: Relative permittivities of binary mixtures of 1-butanol + n-alkane at 298.15 K. J. Therm. Anal. Calorim. 72, 129–133 (2003)
Prezhdo, O.V., Switek, L., Zubkova, V.V., Prezhdo, V.V.: The role of intermolecular interactions in the electro-optical Kerr effect in liquid alkanes. Acta Phys. Pol. A 108, 429–447 (2005)
Sabater, G., Ortega, J.: Excess properties and isobaric vapor–liquid equilibria for four binary systems of alkyl (methyl to butyl) methanoates with decane. Fluid Phase Equilibr. 291, 18–31 (2010)
Vaisarová, V., Chvalovský, V.: Organosilicon compounds. LVI. Dipole moments of substituted vinylsilanes and phenylsilanes. Collect. Czech. Chem. Commun. 33, 859–865 (1968)
Redlich, O., Kister, A.T.: Algebraic representation of thermodynamic properties and the classification of solutions. Ind. Eng. Chem. 40, 345–348 (1948)
Rodríguez, A., Canosa, A.J., Tojo, J.: Binary mixture properties of methyl tert-butyl ether with hexane or heptane or octane or nonane from 288.15 K to 298.15 K. J. Chem. Eng. Data 44, 666–671 (1999)
Domańska, U.: The excess molar volumes of (hydrocarbon + branched chain ether) systems at 298.15 K and 308.15 K, and the application of PFP theory. Fluid Phase Equilib. 130, 207–222 (1996)
Liu, H., Zhu, L.: Excess molar volumes and viscosities of binary systems of butylcyclohexane with n-alkanes (C7 to C14) at T = 293.15 K to 313.15 K. J. Chem. Eng. Data 59, 369–375 (2014)
Kermanpour, F., Niakan, H.Z., Sharifi, T.: Density and viscosity measurements of binary alkanol mixtures from (293.15 to 333.15) K at atmospheric pressure. J. Chem. Eng. Data 58, 1086–1091 (2013)
Peralta, R.D., Infante, R., Cortez, G., Cisneros, A., Wisniak, J.: Densities and excess volumes of benzene with ethyl acrylate, butyl acrylate, methyl methacrylate, and styrene at 298.15 K. Thermochim. Acta 398, 39–46 (2003)
Krakowiak, J., Śmiechowski, M.: Excess molar volume and viscosity deviation for binary mixtures of γ-butyrolactone with dimethyl sulfoxide. J. Chem. Thermodyn. 110, 57–64 (2017)
Gómez, E., González, B., Calvar, N., Tojo, E., Domínguez, Á.: Physical properties of pure 1-ethyl-3-methylimidazolium ethylsulfate and its binary mixtures with ethanol and water at several temperatures. J. Chem. Eng. Data 51, 2096–2102 (2006)
Brocos, P., Piñeiro, Á., Bravo, R., Amigo, A.: Refractive indices, molar volumes and molar refractions of binary liquid mixtures: concepts and correlations. Phys. Chem. Chem. Phys. 5, 550–557 (2003)
Sudhakar, S.D., Chandrashekhar, P.P., Dilip, V.P.: Density, speed of sound, and refractive index of aqueous binary mixtures of some glycol ethers at T = 298.15 K. J. Chem. Eng. Data 55, 3962–3968 (2010)
Acknowledgements
This work was supported by the school-enterprise cooperation project for University Access Engineer of Zhejiang (Grant No. FG2017204).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, J., Shao, F., Dong, H. et al. Volumetric and Optical Properties of Dimethylvinylethoxysilane with n-Alkanes (C7 to C10) at Temperatures in the Range (298.15 to 318.15) K. J Solution Chem 48, 82–103 (2019). https://doi.org/10.1007/s10953-019-00843-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-019-00843-9