Abstract
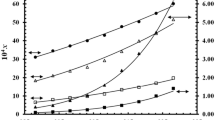
The solubility of telmisartan (form A) in nine organic solvents (chloroform, dichloromethane, ethanol, toluene, benzene, 2-propanol, ethyl acetate, methanol and acetone) was determined by a laser monitoring technique at temperatures from 277.85 to 338.35 K. The solubility of telmisartan (form A) in all of the nine solvents increased with temperature as did the rates at which the solubility increased except in chloroform and dichloromethane. The mole fraction solubility in chloroform is higher than that in dichloromethane, which are both one order of magnitude higher than those in the other seven solvents at the experimental temperatures. The solubility data were correlated with the modified Apelblat equation and λh equations. The results show that the λh equation is in better agreement with the experimental data than the Apelblat equation. The relative root mean square deviations (σ) of the λh equation are in the range from 0.004 to 0.45 %. The dissolution enthalpies, entropies and Gibbs energies of telmisartan in these solvents were estimated by the Van’t Hoff equation and the Gibbs equation. The melting point and the fusion enthalpy of telmisartan were determined by differential scanning calorimetry.





Similar content being viewed by others
References
Patel, P.A., Patravale, V.B.: Commercial telmisartan tablets: a comparative evaluation with innovator brand micardis. Int. J. Pharm. Sci. Res. 1, 282–292 (2010)
Laragh, J.: The renin system and the renal regulation of blood pressure. In: Seldin, D.W., Giebisch, G. (eds.) The Kidney: Physiology and Pathophiyology, 2nd edn, pp. 1411–1453. Raven Press, New York (1992)
Lepek, P., Sawicki, W., Wlodarski, K., Wojnarowska, Z., Paluch, M., Guzik, L.: Effect of amorphization method on telmisartan solubility and the tableting process. Eur. J. Biochem. 83, 114–121 (2013)
Dinnebier, R.E., Sieger, P., Nar, H., Shankland, K., David, W.I.: Structural characterization of three crystalline modifications of telmisartan by single crystal and high-resolution X-ray powder diffraction. J. Pharm. Sci. 89, 1465–1479 (2000)
Adin, I., Iustain, C., Brand, M., Salman, A., Weisman, A.: Processes of Preparing Highly Pure Telmisartan Form A, Suitable for Pharmaceutical Compositions. US Patent 20060276525 (2006)
Donsbach, K., Hof, I.: Crystalline Form of Telmisartan Sodium. US Patent 6737432 (2004)
Chen, Y.: Study on quality control and stability test for telmisartan. Master’s thesis, Zhejiang University (2005)
Park, J., Cho, W., Cha, K., Ahn, J., Han, K., Hwang, S.: Solubilization of the poorly water soluble drug, telmisartan, using supercritical anti-solvent (SAS) process. Int. J. Pharm. 441, 50–55 (2013)
Nývlt, J.: Solid–Liquid Phase Equilibria. Academia Publishing House of the Czechoslovak Academy of Sciences, Prague (1977)
Jiang, Q., Gao, G., Yu, Y., Qin, Y.: Solubility of sodium dimethyl isophthalate-5-sulfonate in water and in water + methanol containing sodium sulfate. J. Chem. Eng. Data 45, 292–294 (2000)
Liu, B., Sun, H., Wang, J., Yin, Q.: Thermodynamic analysis and correlation of solubility of disodium 5′-guanylate heptahydrate in aqueous ethanol mixtures. Fluid Phase Equilib. 370, 58–64 (2014)
Hong, M., Xu, L., Ren, G., Chen, J., Qi, M.: Solubility of lansoprazole in different solvents. Fluid Phase Equilib. 331, 18–25 (2012)
Wei, Y., Zhang, X., Zhang, J., Dang, L., Wei, H.: Solid–liquid equilibrium of some polycyclic aromatic hydrocarbons in wash oil. Fluid Phase Equilib. 319, 23–29 (2012)
Liu, W., Dang, L., Black, S., Wei, H.: Solubility of carbamazepine (form III) in different solvents from (275 to 343) K. J. Chem. Eng. Data 53, 2204–2206 (2008)
Zhang, C., Wang, J., Wang, Y.: Solubility of ceftriaxone disodium in acetone, methanol, ethanol, N, N-dimethylformamide, and formamide between 278 and 318 K. J. Chem. Eng. Data 50, 1757–1760 (2005)
Bradley, J., Abraham, M.H., Acree, W.E., Lang, A.: Predicting Abraham model solvent coefficients. Chem. Cent. J. 9, 1–10 (2015)
Apelblat, A., Manzurola, E.: Solubility of oxalic, malonic, succinic, adipic, maleic, malic, citric, and tartaric acids in water from 278.15 to 338.15 K. J. Chem. Thermodyn. 19, 317–320 (1987)
Buchowski, H., Ksiazczak, A., Pietrzyk, S.: Solvent activity along a saturation line and solubility of hydrogen-bonding solids. J. Phys. Chem. 84, 975–979 (1980)
Mo, Y., Dang, L., Wei, H.: Solubility of α-form and β-form of l-glutamic acid in different aqueous solvent mixtures. Fluid Phase Equilib. 300, 105–109 (2011)
Kai, W., Hu, Y., Yang, W., Song, G., Ying, S.: Measurement and correlation of the solubility of 2,3,4,5-tetrabromothiophene in different solvents. J. Chem. Thermodyn. 55, 50–55 (2012)
Hefter, G.T., Tomkins, R.P.T.: Chapter 1.1. Thermodynamics of Solubility. Wiley, New York (2004)
Ness, H.C.V.: Chemical Thermodynamics, Vol. 2: by M.L. McGlashan (Senior Reporter), The Chemical Society, London, 1978. Fluid Phase Equilib. 2, 312 (1979)
Rodríguez, G.A., Delgado, D.R., Martínez, F., Jouyban, A., Acree, W.E.: Solubility of naproxen in ethyl acetate + ethanol mixtures at several temperatures and correlation with the Jouyban–Acree model. Fluid Phase Equilib. 320, 49–55 (2012)
Panteli, E.K., Voutsas, E.K.: Solubilities of cinnamic acid esters in ionic liquids. J. Chem. Eng. Data 54, 812–818 (2009)
El-Bindary, A.A., El-Sonbati, A.Z., Ahmed, E.M.A.M., El-Bindary, A.A., El-Sonbati, A.Z.: Potentiometric and thermodynamic studies of azosulfonamide drugs. X. Chem. Pap. 57, 255–258 (2003)
Kapil, B., Shipra, B.: Measurement and correlation for solubility of some pyrimidine derivatives in different solvents. J. Appl. Chem. 2014, 1–7 (2014)
Acknowledgments
The authors would like to thank the National Nature and Science Foundation of China (NSFC, No. 21206032), the Science Foundation Henan University of Technology (No. 2012CXRC08) and the Science Foundation of Henan Province (No. 2015GGJS-039) for their financial assistance for this project. Thanks for David Kearns for the language revision.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, W., Guo, Y., Chen, J. et al. Measurement and Correlation of the Solubility of Telmisartan (Form A) in Nine Different Solvents from 277.85 to 338.35 K. J Solution Chem 45, 932–946 (2016). https://doi.org/10.1007/s10953-016-0484-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-016-0484-8