Abstract
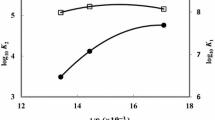
Stoichiometric protonation constants (log10 K 1 and log10 K 2) of some aliphatic dipeptides (Gly–Tyr, Gly–Phe, Gly–Val, Gly–Leu, Gly–Thr, Gly–Met and Gly–Pro) were determined potentiometrically in 20, 40, and 60 % (v/v) 1,4-dioxane–water and dimethyl sulfoxide–water mixtures at 25.0 (±0.1) °C with an ionic strength of 0.10 mol·L−1 sodium chloride. The protonation constants were calculated with the computer program PKAS and selection of the best fit chemical models is based on the statistical parameters. The effects of solvent composition on these protonation constants are discussed to determine the factors which control these processes. It has been observed that, while the correlation between log10 K 1 and log10 K 2 with the percentages of dimethyl sulfoxide in the dimethyl sulfoxide–water mixtures are not linear, these values linearly increase as the concentration of 1,4-dioxane increases in the solvent mixtures.


Similar content being viewed by others
References
Doğan, A., Özel, A.D., Kiliç, E.: The protonation equilibria of selected glycine dipeptides in ethanol–water mixture: solvent composition effect. Amino Acids 36, 373–379 (2009)
Kiani, F., Rostami, A.A., Sharifi, S., Bahadori, A.: Calculation of acidic dissociation constants of glycylglycine in water at different temperatures using ab initio methods. J. Mol. Struct. Theochem. 956, 20–25 (2010)
Namazian, M., Halvani, S.: Calculations of pK a values of carboxylic acids in aqueous solution using density functional theory. J. Chem. Thermodyn. 38, 1495–1502 (2006)
Avdeef, A.: Absorption and Drug Development: Solubility, Permeability, and Charge State. Wiley, New Jersey (2003)
Kozlowski, H., Bal, W., Dyba, M., Kowalil-Jankowska, T.: Specific structure–stability relations in metallopeptides. Coord. Chem. Rev. 184, 319–346 (1999)
Mendieta, J., Diaz-Cruz, M.S., Tauler, R., Esteban, M.: Application of multivariate curve resolution to voltametric data. II. Study of metal-binding properties of the peptides. Anal. Biochem. 240, 134–141 (1996)
Sigel, H. (ed.): Metal Ions in Biological Systems. Marcel Dekker, vol 2, New York (1973)
Blagojevic, V., Chramow, A., Schneider, B.B., Covey, T.R., Bohme, D.K.: Differential mobility spectrometry of ısomeric protonated dipeptides: modifier and field effects on ıon mobility and stability. Anal. Chem. 83, 3470–3476 (2011)
Kilyen, M., Forgo, P., Lakatos, A., Dombi, G., Kiss, T., Kotsakis, N., Salifoglou, A.: Interaction of Al(III) with the peptides AspAsp and AspAspAsp. J. Inorg. Biochem. 94, 207–213 (2003)
Facchin, C., Torre, M.H., Kremer, E., Piro, E.O., Castellano, E.E., Baran, E.J.: Synthesis and characterization of three new dipeptide complexes. J. Inorg. Biochem. 89, 174–180 (2002)
Diaz-Cruz, M.S., Diaz-Cruz, J.M., Mendieta, J., Tauler, R., Esteban, M.: Soft-and hard modeling approaches for the determination of stability constants of metal–peptide systems by voltammetry. Anal. Biochem. 279, 189–201 (2000)
Köseoğlu, F., Kiliç, E., Doğan, A.: Studies on the protonation constants and solvation of α-amino acids in dioxane–water mixtures. Anal. Biochem. 277, 243–246 (2000)
Canel, E., Gültepe, A., Doğan, A., Kiliç, E.: The determination of protonation constants of some amino acids and their esters by potentiometry in different media. J. Solution Chem. 35, 5–19 (2006)
Fiol, S., Brandariz, I., Sasrte de Vicente, M.: The ionization constants of alanine in NaCl at 25 °C Effect of the ionic strength based on three models. Talanta 42, 797–801 (1995)
Partanen, I.J.: Calculation of the first and second stoichiometric dissociation constants of glycine in aqueous sodium chloride solutions at 298.15 K. Ber. Bunsenges. Phys. Chem. 102, 855–864 (1998)
Zekarias, M.T., Hirpaye, B.Y., Rao, G.N.: Effect of dielectric constant on protonation equilibria of glycylglycine in aqueous solutions of propylene glycol and dioxan. Der Pharma Chemica 3, 69–77 (2011)
Catalan, J., Diaz, D., Garcia-Blanco, F.: Characterization of binary solvent mixtures of DMSO with water and other cosolvents. J. Org. Chem. 66, 5846–5852 (2001)
Luzar, A., Stefan, J.: Dielectric behaviour of DMSO–water mixtures. A hydrogen bonding model. J. Mol. Liq. 46, 221–238 (1990)
Mancera, R.L., Chalaris, M., Refsonc, K., Samios, J.: Molecular dynamics simulation of dilute aqueous DMSO solutions. A temperature-dependence study of the hydrophobic and hydrophilic behaviour around DMSO. Phys. Chem. Chem. Phys. 6, 94–102 (2004)
Shashkov, S.N., Kiselev, M.A., Tioutiounnikov, S.N., Kiselev, A.M., Lesieur, P.: The study of DMSO/water and DPPC/DMSO/water system by means of the X-ray, neutron small angle scattering, calorimetry and IR spectroscopy. Phys. B 271, 184–191 (1999)
El-Sherif, A.A., Shoukr, M.M., Abd-Elgawad, M.M.A.: Protonation equilibria of some selected α amino acids in DMSO–water mixture and their Cu(II)-complexes. J. Solution Chem. 42, 412–427 (2013)
Martin, D., Hauthal, H.G.: Dimethylsulphoxide. Van Nostrand Reinhold, Workingham (1975)
Hermandez-Molina, R., Mederos, A., Gili, P., Dominquez, S., Numez, P., Grmain, G., Debaerdemaeker, T.: Coordinating ability in DMSO–water 80:20 wt/wt of the Schiff base N, NO 3,4 toluenebis(salicylideneimine) with divalent cations. Crystal structure of the nickel(II) complex. Inorg. Chim. Acta 256, 319–325 (1997)
Tribolet, R., Malini-Balakrishnan, R., Sigel, H.: Influence of decreasing solvent polarity (dioxane–water mixtures) on the stability and structure of binary and ternary complexes of adenosine 5′-triphosphate and uridine 5′-triphosphate. J. Chem. Soc. Dalton Trans. 11, 2291–2303 (1985)
Doğan, A., Kiliç, E.: Potentiometric studies on the protonation constants and solvation of some α amino acid benzyl- and t-butyl-esters in ethanol–water mixtures. Turk. J. Chem. 20, 41–47 (2005)
Doğan, A., Kiliç, E.: Tautomeric and microscopic protonation constants of amino acids. Anal. Biochem. 365, 7–13 (2007)
Doğan, A., Aslan, N., Canel, E., Kiliç, E.: Solvent effects on the protonation constants of some α amino acid esters in 1,4-dioxane–water mixtures. J. Solution Chem. 39, 1589–1596 (2010)
Gran, G.: Determination of the equivalent point in potentiometric titrations, Part I. Acta Chem. Scand 4, 559–577 (1950)
Gran, G.: Determination of the equivalent point in potentiometric titrations, Part II. Analyst 77, 661–671 (1952)
Martell, A.E., Motekaitis, R.J.: The Determination and use of Stability Constants. VCH, Weinheim (1988)
Meloun, M., Havel, J., Högfelt, H.: Computation of Solution Equilibria. Wiley, New York (1988)
Gündüz, T., Kiliç, E., Canel, E.: Protonation constants of some substituted salicylideneanilines in dioxan–water mixtures. Anal. Chim. Acta 282, 489–495 (1993)
Motekaitis, R.J., Martell, A.E.: Program PKAS: a novel algorithm or the computation of successive protonation constants. Can. J. Chem. 60, 1681–1689 (1982)
Kiliç, E., Aslan, N.: Determination of autoprotolysis constants of water–organic solvent mixtures by potentiometry. Microchim. Acta 151, 89–92 (2005)
Demirelli, H.: On the role of the solvent and substituent on the protonation equilibria of disubstituted anilines in dioxane–water mixed solvents. J. Solution Chem. 34, 1283–1295 (2005)
Jabbari, M., Gharib, F.: Solute–solvent interaction effects on protonation equilibrium of some water-insoluble flavonoids. J. Solution Chem. 40, 561–574 (2011)
Das, A.K., Kundu, K.K.: Autoprotolysis constants of water + dimethyl sulphoxide mixtures at 25 °C and the related free energies of transfer of OH. J. Chem. Soc. Faraday Trans. 1(69), 730–735 (1973)
Jabbari, M., Gharib, F.: Solvent effects on protonation equilibria of some amino acids and peptides in different aqueous solutions of ethanol. Acta Chim. Slov. 57, 325–331 (2010)
Sieler, G., Schweitzer-Stenner, R., Holtz, J.S.W., Pajcini, V., Asher, S.A.: Different conformers and protonation states of dipeptides probed by polarized Raman, UV-resonance Raman and FTIR spectroscopy. J. Phys. Chem. B 103, 372–384 (1999)
Doğan, A., Köseoğlu, F., Kiliç, E.: Studies on the macroscopic protonation constants of some α-amino acids in ethanol–water mixtures. Anal. Biochem. 309, 75–78 (2002)
Taft, R.W., Abboud, J.L.M., Kamlet, M.J.: Linear solvation energy relationships. An analysis of Swain’s solvent “acidity” and “basicity” scales. J. Org. Chem. 49, 2001–2005 (1984)
Kamlet, M.J., Abboud, J.L.M., Abraham, M.H., Taft, R.W.: Linear solvation energy relationships. A comprehensive collection of the solvatochromic parameters, π * , α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 48, 2877–2887 (1983)
Barbosa, J., Barron, D., Beltran, J.L., Buti, S.: On the role of solvent in acid–base equilibria of diuretics in acetonitrile–water mixed solvents. Talanta 45, 807–815 (1998)
Marcus, Y.: The standard partial molar volumes of ions in solution, Part 3. Volumes in solvent mixtures where preferential solvation takes place. J. Solution Chem. 34, 317–331 (2005)
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Erden, P.E., Aslan, N., Doğan, A. et al. Effect of Solvent Composition on Protonation Constants of Some Glycine Dipeptides. J Solution Chem 43, 1156–1166 (2014). https://doi.org/10.1007/s10953-014-0191-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-014-0191-2