Abstract
We present a study on the magnetic properties and magnetocaloric effect (MCE) in R3Co11B4, where R = Pr, Nd, Tb, Dy, and Ho. The two-sublattice model is used for calculating magnetization, magnetic heat capacity, isothermal entropy change ∆Sm, and adiabatic temperature change ∆Tad, for different magnetic field changes ∆H = 1.5, 3, and 5 T and at temperatures up to 600 K. Direct and inverse MCE are shown to take place in the ferrimagnetic compounds with R = Tb, Dy, and Ho. The maximum isothermal magnetic entropy change and maximum adiabatic temperature change have been calculated for ferromagnetic Nd3Co11B4 compound to be 1.85 J/K mol and 6.5 K at Tc = 432 K, for a field change ∆H = 5 T. The relative cooling power (RCP) is in the 44–161 J/mol range for the same field change. Also, the type of phase transition is investigated in the light of Arrott plots, universal curves, and the features of the temperature and field dependences of the magnetization, heat capacity, entropy, and the magnetocaloric properties. Those features confirm that the transition at the Curie temperature of these compounds is of the second order.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The magnetocaloric effect has been discussed before for rare earth inter-metallic compounds [1,2,3]. A large variety of functional materials useful for technological applications such as magnetic refrigeration has been reported. Magnetic refrigeration is hoped to be a good, more efficient, and environmentally safer alternative to traditional refrigeration [4,5,6]. Many studies have been done to investigate the room-temperature magnetocaloric effect (MCE) materials such as La1-xLixMnO3 [7], Gd5Si2Ge2 [8], NiMnIn [9], and NiMnSb [10]. Also, the MEC was studied on other materials such as RCo2 [11], RCuAl [12], RMn2Si2, and HoCoSi [13]. The direct and inverse MCE have been observed in Heusler alloys [14] and ferrimagnetic materials such as RFe2 [15, 16] and DySb [17]. The magnetic properties of R3Co11B4 have been reported by Tetean et al. [18] and Xiang-Mu et al. [19]. In this work, we present a theoretical calculation of the magnetothermal properties and the magnetocaloric effect in R3Co11B4 compounds. In particular, we report on the isothermal change in entropy, the adiabatic change in temperature, and the relative cooling power in fields up to 5 T and at temperatures up to and above the Curie temperature of the compounds under study.
2 Model and Analysis
The exchange field of two sublattices systems can be expressed as follows [20, 21]:
where H is the external applied magnetic field, and MR(T) and MCo(T) are the magnetic moments for rare earth and cobalt, respectively, at temperature T. The factor d = NA ρ μB/A converts the moment per formula unit of R3Co11B4 from μB to A/m, where ρ is the density of the compound in kg/m3, NA is Avogadro’s number, and A is a formula unit weight of compound in kg per mole. The molecular field coefficients nRR, nCoCo, and nRCo are dimensionless.
MCo(0) and MR(0) are the magnetic moments of cobalt and rare earth at T = 0 K, respectively, and MR(0) = gR JR, where g and J have their usual meaning. Mco(0) is, however, obtained from the experimental low temperature data.
BJ(y) is the Brillouin function:
and \(\mathrm{y}=\frac{\mathrm{\mu H}}{\mathrm{kT}}\)
The total magnetization can be calculated from:
The magnetic entropy change is calculated from the thermodynamic Maxwell relation:
The magnetic entropy change is calculated also by using trapezoidal rule, as reported, for example, by Tishin et al. [22].
where M(H) is the isothermal magnetization.
A universal curve [23] is the relation between △Sm/△\({\mathrm{S}}_{\mathrm{m}}^{\mathrm{peak}}\) and ϴ, where ϴ is defined as follows:
One may choose the reference temperature Tr such that [23]:
The total heat capacity Ctot includes three contributions: the magnetic heat capacity Cm, the electronic heat capacity Ce, and the lattice heat capacity Cl [24, 25]:
First, we calculate the magnetic energy for the system in order to calculate the magnetic contribution to heat capacity:
The magnetic contribution to heat capacity is calculated from the temperature-derivative of magnetic energy:
Second, the electronic contribution to heat capacity is proportional to temperature and may become a dominant term at very low temperatures; the electronic heat capacity is given by [26]:
where γe is the electronic heat-capacity coefficient and N (Ef) is the density-of-states at Fermi energy.
Third, the lattice contribution to heat capacity is expressed in the Debye model, as follows:
where x = ϴD/T and ϴD is Debye temperature.
The adiabatic temperature change [24] can be calculated from the following:
According to Arrot-Belov-Kouvel (ABK) [27, 28], the Arrott plots M2 vs. H/M and M2 vs. ∆Sm in the ferromagnetic region at different temperatures, close to Tc, can be used to estimate the spontaneous magnetization and the Curie temperature.
From Landau-Ginsburg theory [29], Gibb’s free energy is expressed as follows:
The magnetic entropy change obtained from Gibb’s free energy:
From the equilibrium condition at Tc, \(\frac{\partial \mathrm{F}}{\partial \mathrm{M}}=0\) the magnetic equation of state is:
where A(T) and B(T) are Landau’s coefficients.
The relative cooling power (RCP) [30] is defined as follows:
where ∆Smax (T) is the maximum magnetic entropy change and δTFWHM is the full width at half maximum of the magnetic entropy change curve.
3 Results and Discussion
3.1 Magnetization
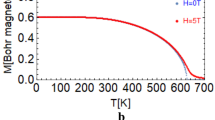
In this study, we calculated the temperature dependences of the magnetization of the rare-earth, Co, and R3Co11B4 system, where R = Pr, Nd, Tb, Dy, and Ho. Ferromagnetic coupling is present in compounds with R = Nd and Pr, whereas compounds with R = Tb, Dy, and Ho show ferrimagnetic coupling with compensation points. Figure 1(a) exhibits the magnetization of the two sublattices of Nd and Co, and the total magnetization of the compound. Figure 1(b) shows the same but for the ferrimagnetic compound with R = Dy where a compensation point is present. We may remark that the magnetic moments, at 0 K, for the Nd and Dy atoms are as follows: MNd (0) = gNd × JNd = (8/11) × (7/2) = 2.54 μB/atom and MDy (0) = gDy × JDy = (4/3) × (15/2) = 10 μB/atom. It is known that the nature of the magnetic moment in R-atoms is localized whereas that of the 3d-transition elements (Co in the present study) is relatively itinerant. Therefore, the magnetic moment of the cobalt sublattice is determined from the experimental data of the total magnetization and from the localized moments of the R-elements, as shown above. In the Nd compound, the cobalt moment per atom is MCo(0) = 4.4/11 = 0.4 μB, but it is different in the Dy compound, i.e., MCo(0) = 13.36/11 = 1.214 μB [19]. We may recall that, in the elemental cobalt, the magnetic moment of a Co atom, at 0 K, is 1.7 μB; however, it might be drastically different in some R-Co compounds; e.g., in RCo2 compounds, the Co moment vanishes [31]. For the elemental Dy, however, MDy (0) = 10 μB, this same value of MDy (0), in the Dy3Co11B4 compound, led to the observed experimental total magnetization of the compound.
Results of total magnetization and Curie temperatures, for the R3Co11B4 system, are shown in Table 1. In this table, the percentage difference between our calculated magnetic moment, at very low temperatures, and the experimentally determined moments [18] is displayed. This difference is only ≤ 0.6%. The corresponding difference in the Curie temperature data is ≤ 7%. We may also refer to the value of the magnetic moment of the Nd3Co11B4 compound, as evaluated by the band calculation [32], i.e., 15.87 µB/f.u which is about 30% off the experimental value 12.2 µB/f.u reported in the same reference. It is worth mentioning that, in our mean-field calculation of M (T, H), we picked up initial magnetic moment values, at temperatures (with a step of 1 K) up to Curie temperature and in a specific field, and checked, in an iteration process, whether they are possible simultaneous solutions to the well-known Brillouin functions. This iteration process was performed with an accuracy of 10−7.
3.2 Total Heat Capacity
Basically, the calculation of the adiabatic change in temperature depends on both of the temperature change of the magnetization and total heat capacity (Eq. 16). There are three contributions to the total heat capacity for R3Co11B4, as we mentioned before in Eq. (11). First, the magnetic heat capacity has been calculated from Eq. (13), and is shown, for different magnetic fields: 0, 1.5, 3, and 5 T, for Nd3Co11B4 and Ho3Co11B4 in Fig. 2a and b, respectively. Second, electronic heat capacity is calculated from the coefficient γe, which is obtained from the materials project [33], A Szajek [34], and A Kowalczyk et al. [32] as shown in Table 2. The Debye temperatures of Nd3Co11B4 and Gd3Co11B4 are 475 and 450 K, respectively, as reported by Li et al. [35]. Debye temperatures for the rest of the systems we report on here are not available and we assumed that their Debye temperature is around 450 K.
3.3 The Isothermal Entropy Change
The isothermal change in entropy has been calculated by two methods: by Maxwell’s relation (Eq. 7) and by the trapezoidal method (Eq. 8). Figure 3(a) shows the isothermal magnetic entropy change for R = Nd. Only direct MCE is present in this (and in the R = Pr) ferromagnetic system. For the ferrimagnetic compound with R = Dy, the isothermal magnetic entropy, for different magnetic field changes, is shown in Fig. 3(b) and it exhibits both direct and inverse MCE; i.e., two peaks are present: the first one at the Curie temperature, and the second one at a temperature below the compensation temperature. Comparing data of △Sm, using Maxwell relation and the trapezoidal method, showed a reasonable agreement between the two methods, in particular for small field changes (Table 2). In the trapezoidal method, which casts the Maxwell integral into a summation, we chose small field changes in order for the summation to be as accurate as possible. For the sake of comparison with bench-mark materials and other R3Co11B4 compounds, e.g., (GdxY1-x)3Co11B4, we compare our results of ΔSm, which is in the range 0.7 to 2.5 J/ mol K at field 5 T, with that of Gd metal, i.e., 1.48 J/mol K at H = 5 T as reported by Wang et al. [36] and with the work of Burzo et al. on (GdxY1-x)3Co11B4 [37], who reported a ΔSm about 2.4 J/mol K at ΔH = 3 T.
3.4 Adiabatic Temperature Change
In this part, we report on the adiabatic temperature change. Because of a weak dependence of the total heat capacity on the applied magnetic field, around Tc for the studied compounds, for example, for R = Dy (Fig. 4), the term T/\({\mathrm{C}}_{\mathrm{tot}}\) is taken out of the integral in Eq. 16. Figure 5(a and b) shows the adiabatic temperature change for R = Nd and Dy, respectively. The results, for four studied compounds, are shown in Table 3. The maximum value for ΔT is 6.5 K, for an applied magnetic field 5 T, in case of Nd3Co11B4; i.e., the temperature is decreasing by a rate of 1.3 K/T.
3.5 The Relative Cooling Power
The relative cooling power is a figure-of-merit in the field of magnetic refrigeration and is calculated, for different field changes by Eq. (21). The RCP increases with increasing the applied magnetic field, and the results are summarized in Table 4. The RCP is in the range 17.48 to 160.95 J/ mol at field 5 T, as compared to about 108.33 J/ mol at 5 T for Gd as reported by Wang et al. [36].
3.6 Arrott Plots
Figures 6(a, b) and 7(a, b) show (H/M) vs. M2 and ∆Sm vs. M2 plots for R = Nd and Dy, respectively, in different magnetic fields, and at temperatures close to Tc. The positive slopes of these plots indicate a second-order phase transition. The curve starting from the origin represents the data at Tc and this is in agreement with temperature dependence of magnetization, according to the Banerjee criterion [28].
3.7 Universal Curve
Figure 8 shows the universal curve of Dy3Co11B4 in applied magnetic fields of 1.5, 3, and 5 T. The normalized magnetic entropy change curves fairly collapse onto a unique curve, which confirms that the phase transition in the R3Co11B4 system is of the second order.
3.8 The Field-Dependence of the Magnetic Entropy Change
Figure 9 shows the field dependence of the magnetic entropy change for R3Co11B4 system. According to the MFT, the relation ΔSm vs. (H/TC)2/3 is another criterion for existence of a second-order magnetic phase transition [38, 39].
4 Conclusion
We calculated the magnetothermal and MC properties, △Sm and △Tad for R3Co11B4 system using the mean-field theory. Magnetization calculations showed that compounds with R = Tb, Dy, and Ho are ferrimagnetic compounds, whereas those with R = Pr and Nd are ferromagnetic. △Sm is calculated by two methods: Maxwell’s relation and the trapezoidal method. The two methods give fairly similar results. The highest ordinary MCE △Sm, △Tad, and RCP are 2.5 J/mol K, 6.6 K, and 161 J/mol for R = Nd for a magnetic field change of 5 T. The temperature and field dependencies of the magnetic properties, △Sm, △Tad, Arrott plots, and the universal curves showed that the phase transition in these compounds is of the second order. The mean-field analysis is suitable for studying the magnetothermal properties and magnetocaloric effect of the R3Co11B4 system.
References
Andreenko, A.S., Nikitin, S.A., Tishin, A.M.: Magnetocaloric effects in rare-earth magnetic materials. Sov. Phys. USP. 32, 649 (1989). https://doi.org/10.1070/PU1989v032n08ABEH002745
Gschneidner, K.A., Jr., Pecharsky, V.K.: Thirty years of near room temperature magnetic cooling. Int. J. Refrig. 31, 945–961 (2008). https://doi.org/10.1016/j.ijrefrig.2008.01.004
Brück, E.: Developments in magnetocaloric refrigeration. J. Phys. D: Appl. Phys. 38, 381 (2005). https://doi.org/10.1088/0022-3727/38/23/R01
Raghu Ram, N., Prakash, M., Naresh, U., Kumar, N.S., Sarmash, T.S., Subbarao, T., Jeevan Kumar, R., Kumar, G.R., Babu Naidu, K.C.: Review on magnetocaloric effect and materials. J. Supercond. Nov. Magn. 31, 1971–1979 (2018). https://doi.org/10.1007/s10948-018-4666-z
Pecharsky, V.K., Gschneidner, Jr, K.A.: Intermetallic compounds: principles and practice, ch.5. Magnetic reifrigration, John Wiley & Sons (2002). https://doi.org/10.1002/0470845856.ch25
Tishin, A.M., Spichkin, Y.I.: Recent progress in magnetocaloric effect: Mechanisms and potential applications. Int. J. Refrig. 37, 223–229 (2014). https://doi.org/10.1016/j.ijrefrig.2013.09.012
El-Sayed, A.H., Hamad, M.A.: Magnetocaloric effect in La1−xLixMnO3. J. Supercond. Nov. Magn. 31, 4167–4171 (2018). https://doi.org/10.1007/s10948-018-4699-3
Jabrane, M., El Hafidi, M.Y., El Hafidi, M.: Magnetocaloric properties of Gd5Si2Ge2 alloy: Monte Carlo simulation study. J. Supercond. Nov. Magn. 32, 2579–2587 (2019). https://doi.org/10.1007/s10948-018-4992-1
Moya, M., Mañosa, L., Planes, A., Aksoy, S., Acet, M., Wassermann, E.F., Krenke, T.: Cooling and heating by adiabatic magnetization in the NiMnIn magnetic shape-memory alloy. Phys. Rev. B. 75, 184412 (2007). https://doi.org/10.1103/PhysRevB.75.184412
Nayak, A.K., Suresh, K.G., Nigam, A.K.: Giant inverse magnetocaloric effect near room temperature in Co substituted NiMnSb Heusler alloys. J. Phys. D. 42, 035009 (2009). https://doi.org/10.1088/0022-3727/42/3/035009
Duc, N.H., Anh, D.T.K., Brommer, P.E.: Metamagnetism, giant magnetoresistance and magnetocaloric effects in RCo2-based compounds in the vicinity of the Curie temperature. Phys. B. 1, 319 (2002). https://doi.org/10.1016/S0921-4526(02)01099-2
Dong, Q.Y., Chen, J., Shen, J., Sun, J.R., Shen, B.G.: Large magnetic entropy change and refrigerant capacity in rare-earth intermetallic RCuAl (R=Ho and Er) compounds. J. Magn. Magn. Mater. 324, 2676 (2012). https://doi.org/10.1016/j.jmmm.2012.03.052
Dos Reis, D.C., França, J.K.P., Andrade-Araujo, R., Dos Santos, A.O., Coelho, A.A., Cardoso, L.P., Da Silva, L.M.: The wide operating temperature range in the magnetocaloric composite formed by RMn2Si2 (R=Tm, Tb, and Dy) and HoCoSi Compounds. J. Supercond. Nov. Magn. 33, 3773–3780 (2020). https://doi.org/10.1007/s10948-020-05644-6
Zhukova, V., Aliev, A., Varga, R., Aronin, A., Abrosimova, G., Kiselev, A., Zhukov, A.: Magnetic properties and MCE in Heusler-type glass-coated microwires. J. Supercond. Nov. Magn. 26, 1415–1419 (2013). https://doi.org/10.1007/s10948-012-1978-2
Germano, D., Butera, R.: Heat capacity and thermodynamic functions of the RFe2 compounds (R = Gd, Tb, Dy, Ho, Er, Tin, Lu) over the Temperature Region 8 to 300 K. Phys. Rev. B. 24, 3912 (1981). https://doi.org/10.1016/0022-4596(81)90500-4
Nagy, A., Hammad, T., Yehia, S., Aly, S.H.: Thermomagnetic properties and magnetocaloric effect of TmFe2 compound. J. Magn. Magn. Mater. 10, 50 (2018). https://doi.org/10.1016/j.jmmm.2018.10.050
Hu, W.J., Du, J., Li, B., Zhang, Q., Zhang, Z.D.: Giant magnetocaloric effect in the Ising anti- ferromagnet DySb. Appl. Phys. Lett. 92, 192505 (2008). https://doi.org/10.1063/1.2928233
Tetean, R., Burzo, E.: Magnetic properties of R3Co11B4 compounds. J. Magn. Magn. Mater. 157, 633 (1996). https://doi.org/10.1016/0304-8853(95)01034-3
Xiang-Mu, Z., Rui-Wang, H., Zhong-Wu, Z.: Molecular field theory analysis of R3Co11B4 compounds. J. Magn. Magn. Mater. 241, 131–136 (2002). https://doi.org/10.1016/S0304-8853(01)01051-4
Herbst, J., Croat, J.: Magnetization of R6Fe2 3 intermetallic compounds: molecular field theory analysis. J. Appl. Phys. 55, 3023–3027 (1984). https://doi.org/10.1063/1.333293
Khedr, D.M., Aly, S.H.M., Shabara, R.M., Yehia, S.: A molecular-field study on the magnetocaloric effect in Er2Fe17. J. Magn. Magn. Mater. 475, 436–444 (2019). https://doi.org/10.1016/j.jmmm.2018.11.079
Tishin, A.M., Spichkin, Y.I.: The Magnetocaloric effect and its applications. 1–3 (2003). ISBN: 0–7503–0922–9. https://doi.org/10.1201/9781420033373
Franco, V., Conde, A.: Scaling laws for the magnetocaloric effect in second order phase transitions. Int. J. Refrig. 33(3), 465–473 (2010). https://doi.org/10.1016/j.ijrefrig.2009.12.019
de Oliveira, N.A., Von Ranke, P.J.: Theoretical aspects of the magnetocaloric effect. Phys. Rep. 489, 89–159 (2010). https://doi.org/10.1016/j.physrep.2009.12.006
Debye, P.: On the theory of specific heats. Ann. Phys. 344, 789–839 (1912). https://doi.org/10.1002/andp.19123441404
Kittel, C.: Introduction to solid state physics, 7 edition, John Wiley &Sons (1996). ISBN: 0–471–11181–3
Yeung, I., Roshko, R.M., Williams, G.: Arrott-plot criterion for ferromagnetism in disordered systems. Phys. Rev. B. 34, 3456–3457 (1986). https://doi.org/10.1103/PhysRevB.34.3456
Banerjee, B.K.: On a generalized approach to first and second order magnetic transitions. Phys. Lett. 12, 16–17 (1964). https://doi.org/10.1016/0031-9163(64)91158-8
Amaral, V.S., Amaral, J.S.: Magnetoelastic coupling influence on the magnetocaloric effect in ferromagnetic materials. J. Magn. Magn. Mater. 272, 2104 (2004). https://doi.org/10.1016/j.jmmm.2003.12.870
Franco, V., Blázquez, J.S., Ingale, B., Conde, A.: The magnetocaloric effect and magnetic refrigeration near room temperature: materials and models. Annu. Rev. Mater. Res. 42, 305–342 (2012). https://doi.org/10.1146/annurev-matsci-062910-100356
Cyrot, M., Gignoux, D.M., Givord, F., Lavagna. M.: Magnetism of rare-earth 3d: a theoretical review. J. de Physique Colloque C5, C5–171 (1979). https://doi.org/10.1051/jphyscol:1979563
Kowalczyk, A., Jezierski, A.: Electron-transport properties and electronic structure of the Nd3Co11B4 compound. J. Magn. Magn. Mater. 182, 137–142 (1998). https://doi.org/10.1016/S0304-8853(97)00742-7
Persson and Kristin, Materials Project. (2016). https://doi.org/10.17188/1267087, https://doi.org/10.17188/1205255
Szajek, A., Morkowski, J.A.: Calculated magnetic moments and electronic structures of the compound Rn+1Co3n+5B2n, R = Gd, Tb. Mater. Sci.-Poland. 24, 839 (2006)
Li, J.C., Qian, P., Zhang, F.Z., Liu, Y., Wang, Y.W., Shen, J., Chen, N.: X, Theoretical study of structure and lattice vibrations of R3Co11−xFexB4 (R=Nd, Gd). Comput. Phys. Commun. 184, 342–347 (2013). https://doi.org/10.1016/j.cpc.2012.09.017
Wang, G.F., Li, L.R., Zhao, Z.R., Yu, X.Q., Zhang, X.F.: Structural and magnetocaloric effect of Ln0.67Sr0.33MnO3 (Ln=La, Pr and Nd) nanoparticles. J. Ceram. Int. 40, 16449–16454 (2014). https://doi.org/10.1016/j.ceramint.2014.07.154
Burzo, E., Codescub, M.M., Kappeld, W., Helerarea, E.: Magnetic materials for technical applications. J. Optoelectron. Adv. M. 11, 229–237 (2009)
Burrola, L.A., Andara, G., Rodriguez, C.R., Gomez, F.J., Saenz-Hernandez, R.J., Zubiate, M.E., Aquino, A.: Comparison of the order of magnetic phase transitions in several magnetocaloric materials using the rescaled universal curve, Banerjee and mean field theory criteria. J. Appl. Phys. 117, 144 (2015). https://doi.org/10.1063/1.4918340
Belo, J.H., Amaral, J.S., Pereira, A.M., Amaral, V.S., Araul, J.P.: On the Curie temperature dependency of the magnetocaloric effect. Appl. Phys. Lett. 100, 242407 (2012). https://doi.org/10.1063/1.4726110
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abu Elnasr, R., Aly, S.H., Yehia, S. et al. Magnetothermal Properties and Magnetocaloric Effect in R3Co11B4. J Supercond Nov Magn 35, 2555–2562 (2022). https://doi.org/10.1007/s10948-022-06298-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-022-06298-2