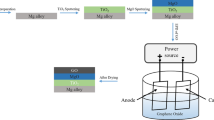
Abstract
Porous-Ti64 alloys (P-Ti64), produced at various porosities by hot-pressing technique with the help of Mg spacer, were coated by hybrid-Graphene Oxide (rGO) reinforced-hydroxyapatite (HAp), using the sol–gel method. The synthesized rGO powder was used in reinforcing HAp by the Modified Hummers method having 30 µm particle size and nano (nm) scale layer thickness. Hybrid coatings were executed on Ti64 substrates in four different groups as single-HAp, HAp reinforced with 0.5 wt%, 1.0 wt% and 1.5 wt% rGO for three different porosities (41, 52, and 64%) were characterized by FT-IR, Raman, XRD and SEM. The average 21 µm coating film thicknesses were obtained and desirably, the only superficial pores of the substrates were closed by coating material rather than the inner connected open pores. It was also shown that 0.5 wt% and 1.0 wt% rGO reinforcements into HAp prevented crack formation on the Ti64 surfaces, whereas 1.5 wt% rGo reinforcement and single-HAp coatings caused. The highest adhesion strength values were achieved at low porosities (41–52%) and of 0.5–1.0 wt% rGO reinforcements through the adhesion tests.














Similar content being viewed by others
References
D. Shi, Biomaterials and Tissue Engineering (Springer-Verlag, Berlin Heidelberg, 2004).
H. Wang, C. Lin, R. Hu, Effects of structure and composition of the CaP composite coatings on apatite formation and bioactivity in simulated body fluid. Appl. Surf. Sci. 255, 4074–4081 (2009). https://doi.org/10.1016/j.apsusc.2008.10.111
B. Brown, P.W. Constantz, Hydroxyapatite and Related Materials (CRC Press, New York, 2017).
J. Xiong, Y. Li, X. Wang, P. Hodgson, C. Wen, Mechanical properties and bioactive surface modification via alkali-heat treatment of a porous Ti–18Nb–4Sn alloy for biomedical applications. Acta Biomater. 4, 1963–1968 (2008). https://doi.org/10.1016/j.actbio.2008.04.022
C.E. Wang, X.J. Xiong, J.Y. Li, Y.C. Hodgson, P.D. Wen, Apatite formation on nano-structured titanium and niobium surface. Mater. Sci. Forum. 614, 85–92 (2009)
Q. Zhang, Y. Leng, R. Xin, A comparative study of electrochemical deposition and biomimetic deposition of calcium phosphate on porous titanium. Biomaterials 26, 2857–2865 (2005). https://doi.org/10.1016/j.biomaterials.2004.08.016
I.S. Park, T.G. Woo, W.Y. Jeon, H.H. Park, M.H. Lee, T.S. Bae, K.W. Seol, Surface characteristics of titanium anodized in the four different types of electrolyte. Electrochim. Acta. 53, 863–870 (2007). https://doi.org/10.1016/j.electacta.2007.07.067
W. Jing, M. Zhang, L. Jin, J. Zhao, Q. Gao, M. Ren, Q. Fan, Assessment of osteoinduction using a porous hydroxyapatite coating prepared by micro-arc oxidation on a new titanium alloy. Int. J. Surg. 24, 51–56 (2015). https://doi.org/10.1016/j.ijsu.2015.08.030
X. Chen, Y. Li, P.D. Hodgson, C. Wen, Microstructures and bond strengths of the calcium phosphate coatings formed on titanium from different simulated body fluids. Mater. Sci. Eng. C. 29, 165–171 (2009). https://doi.org/10.1016/j.msec.2008.06.004
C. Domínguez-Trujillo, E. Peón, E. Chicardi, H. Pérez, J.A. Rodríguez-Ortiz, J.J. Pavón, J. García-Couce, J.C. Galván, F. García-Moreno, Y. Torres, Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coatings Technol. 333, 158–162 (2018). https://doi.org/10.1016/j.surfcoat.2017.10.079
J. Mediaswanti, K. Wen, C. Ivanova, E.P. Berndt, C.C. Pham, V.T. Malherbe, F. Wang, Investigation of bacterial attachment on hydroxyapatite-coated titanium and tantalum. Int. J. Surf. Sci. Eng. 15, 255–263 (2014)
B. Aksakal, A.R. Boccaccini, Electrophoretic deposition of selenium. Mater. Lett. 76, 177–180 (2012). https://doi.org/10.1016/j.matlet.2012.02.059
B. Aksakal, M. Kom, H.B. Tosun, M. Demirel, Influence of micro- and nano-hydroxyapatite coatings on the osteointegration of metallic (Ti6Al4 V) and bioabsorbable interference screws: An in vivo study. Eur. J. Orthop. Surg. Traumatol. 24, 813–819 (2014). https://doi.org/10.1007/s00590-013-1236-8
R.I.M. Asri, W.S.W. Harun, M.A. Hassan, S.A.C. Ghani, Z. Buyong, A review of hydroxyapatite-based coating techniques: Sol–gel and electrochemical depositions on biocompatible metals. J. Mech. Behav. Biomed. 57, 95–108 (2016). https://doi.org/10.1016/j.jmbbm.2015.11.031
X. Pang, I. Zhitomirsky, Electrodeposition of hydroxyapatite–silver–chitosan nanocomposite coatings. Surf. Coat. Technol. 202, 3815–3821 (2008). https://doi.org/10.1016/j.surfcoat.2008.01.022
X. Ji, W. Lou, Qi. Wang, J. Ma, H. Xu, Q. Bai, C. Liu, Liu, Sol-gel-derived hydroxyapatite-carbon nanotube/titania coatings on titanium substrates. Int. J. Mol. Sci. 13, 5242–5253 (2012)
S. Baradaran, E. Moghaddam, W.J. Basirun, M. Mehrali, M. Sookhakian, M. Hamdi, M.R.N. Moghaddam, Y. Alias, Mechanical properties and biomedical applications of a nanotube hydroxyapatite-reduced graphene oxide composite. Carbon N. Y. 69, 32–45 (2014). https://doi.org/10.1016/j.carbon.2013.11.054
L. Zhang, W. Liu, C. Yue, T. Zhang, P. Li, Z. Xing, Y. Chen, A tough graphene nanosheet/hydroxyapatite composite with improved in vitro biocompatibility. Carbon N. Y. 61, 105–115 (2013). https://doi.org/10.1016/j.carbon.2013.04.074
Y. Bai, Y. Bai, J. Gao, W. Ma, J. Su, R. Jia, Preparation and characterization of reduced graphene oxide/fluorhydroxyapatite composites for medical implants. J. Alloys Compd. 688, 657–667 (2016). https://doi.org/10.1016/j.jallcom.2016.07.006
E. Bulbul, B. Aksakal, Synthesizing and characterization of nano-graphene oxide-reinforced hydroxyapatite coatings on laser treated Ti6Al4V surfaces. Acta Bioeng. Biomech. 19, 171–180 (2014). https://doi.org/10.5277/ABB-00876-2017-03
E. Yılmaz, B. Çakıroğlu, A. Gökçe, F. Findik, H.O. Gulsoy, N. Gulsoy, Ö. Mutlu, M. Özacar, Novel hydroxyapatite/graphene oxide/collagen bioactive composite coating on Ti16Nb alloys by electrodeposition. Mater. Sci. Eng. C. 101, 292–305 (2019). https://doi.org/10.1016/j.msec.2019.03.078
W.S. Hummers, R.E. Offeman, Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958). https://doi.org/10.1021/ja01539a017
S. Park, J. An, J.R. Potts, A. Velamakanni, S. Murali, R.S. Ruoff, Hydrazine-reduction of graphite- and graphene oxide. Carbon N. Y. 49, 3019–3023 (2011). https://doi.org/10.1016/j.carbon.2011.02.071
S. Pei, H.-M. Cheng, The reduction of graphene oxide. Carbon N. Y. 50, 3210–3228 (2012). https://doi.org/10.1016/j.carbon.2011.11.010
M. Wojtoniszak, X. Chen, R.J. Kalenczuk, A. Wajda, J. Łapczuk, M. Kurzewski, M. Drozdzik, P.K. Chu, E. Borowiak-Palen, Synthesis, dispersion, and cytocompatibility of graphene oxide and reduced graphene oxide. Colloids Surf. B 89, 79–85 (2012). https://doi.org/10.1016/j.colsurfb.2011.08.026
H. Feng, R. Cheng, X. Zhao, X. Duan, J. Li, A low-temperature method to produce highly reduced graphene oxide. Nat. Commun. 4, 1539 (2013). https://doi.org/10.1038/ncomms2555
D. Konios, M.M. Stylianakis, E. Stratakis, E. Kymakis, Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 430, 108–112 (2014). https://doi.org/10.1016/j.jcis.2014.05.033
A.C. Ferrari, J.C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K.S. Novoselov, S. Roth, A.K. Geim, Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 97, 187401 (2006). https://doi.org/10.1103/PhysRevLett.97.187401
W. Chen, L. Yan, P.R. Bangal, Chemical Reduction of Graphene Oxide to Graphene by Sulfur-Containing Compounds. J. Phys. Chem. C. 114, 19885–19890 (2010). https://doi.org/10.1021/jp107131v
B. Aksakal, M. Demirel, The effect of Zirconia/Yttria/Silver substitutions on mechanostructure and cell viability of the synthesized bioceramic bone grafts. Ceram. Int. 43, 7482–7487 (2017). https://doi.org/10.1016/j.ceramint.2017.03.026
F. Songur, B. Dikici, M. Niinomi, E. Arslan, The plasma electrolytic oxidation (PEO) coatings to enhance in-vitro corrosion resistance of Ti–29Nb–13Ta–4.6Zr alloys: The combined effect of duty cycle and the deposition frequency. Surf. Coatings Technol. 374, 345–354 (2019). https://doi.org/10.1016/j.surfcoat.2019.06.025
Y. Say, B. Aksakal, Enhanced corrosion properties of biological NiTi alloy by hydroxyapatite and bioglass based biocomposite coatings. J. Mater. Res. Technol. 9, 1742–1749 (2020). https://doi.org/10.1016/j.jmrt.2019.12.005
A.-R. Ibrahim, X. Li, Y. Zhou, Y. Huang, W. Chen, H. Wang, J. Li, Synthesis of spongy-like mesoporous hydroxyapatite from raw waste eggshells for enhanced dissolution of ibuprofen loaded via supercritical CO2. Intern. J. Mol. Sci. 16, 7960–7975 (2015). https://doi.org/10.3390/ijms16047960
F. Bakan, O. Laçin, H. Sarac, A novel low temperature sol–gel synthesis process for thermally stable nano crystalline hydroxyapatite. Powder Technol. 233, 295–302 (2013). https://doi.org/10.1016/j.powtec.2012.08.030
C.Y. Ooi, M. Hamdi, S. Ramesh, Properties of hydroxyapatite produced by annealing of bovine bone. Ceram. Int. 33, 1171–1177 (2007). https://doi.org/10.1016/j.ceramint.2006.04.001
P. Wang, C. Li, H. Gong, X. Jiang, H. Wang, K. Li, Effects of synthesis conditions on the morphology of hydroxyapatite nanoparticles produced by wet chemical process. Powder Technol. 203, 315–321 (2010). https://doi.org/10.1016/j.powtec.2010.05.023
V. Miskovic-Stankovic, S. Erakovic, A. Jankovic, M. Vukašinović-Sekulić, M. Mitrić, Y.-C. Jung, S.-J. Park, K.-Y. Rhee, Electrochemical synthesis of nanosized hydroxyapatite/graphene composite powder. Carbon Lett. 16, 233–240 (2015). https://doi.org/10.5714/CL.2015.16.4.233
H.-W. Kim, Y.-H. Koh, L.-H. Li, S. Lee, H.-E. Kim, Hydroxyapatite coating on titanium substrate with titania buffer layer processed by sol–gel method. Biomaterials 25, 2533–2538 (2004). https://doi.org/10.1016/j.biomaterials.2003.09.041
S. Zhang, Y.S. Wang, X.T. Zeng, K.A. Khor, W. Weng, D.E. Sun, Evaluation of adhesion strength and toughness of fluoridated hydroxyapatite coatings. Thin Solid Films 516, 5162–5167 (2008). https://doi.org/10.1016/j.tsf.2007.07.063
K. Cheng, C. Ren, W. Weng, P. Du, G. Shen, G. Han, S. Zhang, Bonding strength of fluoridated hydroxyapatite coatings: A comparative study on pull-out and scratch analysis. Thin Solid Films 517, 5361–5364 (2009). https://doi.org/10.1016/j.tsf.2009.03.122
Cook, K.A. Thomas, J.F. Kay, M. Jarcho. Hydroxyapatite-coated porous titanium for use as an orthopedic biologic attachment system. Clin. Orthopaedic. Relat. Res. 303–312. (1988) http://europepmc.org/abstract/MED/2835198.
B. Aksakal, C. Hanyaloglu, Bioceramic dip-coating on Ti–6Al–4V and 316L SS implant materials. J. Mater. Sci. Mater. Med. 19, 2097–2104 (2008). https://doi.org/10.1007/s10856-007-3304-2
Author information
Authors and Affiliations
Contributions
NA contributing the tests and characterization analysis. BA Conducting-supervising the research and writing the paper.
Corresponding author
Ethics declarations
Conflict of interest
The work content has not been published yet before; it is not under consideration for publication anywhere else. The authors declare that they have no any competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Behalf of authors: Prof B. Aksakal
Rights and permissions
About this article
Cite this article
Aslan, N., Aksakal, B. Effect of graphene reinforcement on hybrid bioceramic coating deposited on the produced porous Ti64 alloys. J Porous Mater 28, 1301–1313 (2021). https://doi.org/10.1007/s10934-021-01081-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-021-01081-5