Abstract
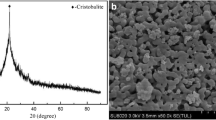
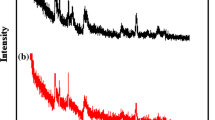
A nearly pure, pale brown, spherical, nitrate cancrinite zeolite was successfully synthesized from natural bentonite as starting material with acidic activation treatment via hydrothermal method at 368 K for 24 h. The effect of different NaOH concentrations (pH at around 12) was investigated without addition of silica and aluminum sources. The effect of different NaOH concentrations (pH at around 12) was investigated without addition of silica and aluminum sources. The final products were characterized by powder X-ray diffraction, scanning electron microscopy, elemental and thermal analyses, infrared (IR) spectroscopy, and Brunauer–Emmett–Teller (BET) surface area measurements. While the Si/Al ratio of ideal cancrinite is 1.0, the Si/Al ratio of the product framework is approximately 1.76, apart from trace components. Unique single nitrate band was observed in both IR and thermogravimetric measurements, indicating that pure cancrinite was synthesized. This study showed that pure nitrate cancrinite was obtained with NaOH concentrations from 8 to 12 M, independent of NaOH contents on crystallization. Through this study, we proposed a simple synthesis method for pure nitrate cancrinite from bentonite for the purpose of recycling natural clay minerals.




Similar content being viewed by others
References
D.W. Breck, Zeolite Molecular Sieves. (Wiley, New York, 1974)
A. Chaisena, K. Rangsriwatananon, Mater. Lett. 59, 1474–1479 (2005)
H. Faghihian, N. Godazandeha, J. Porous Mater. 16, 331–335 (2009)
H. Ma, Q. Yao, Y. Fu, C. Ma, X. Dong, Ind. Eng. Chem. Res. 49, 454–458 (2010)
C. Chen, D.-W. Park, W.-S. Ahn, Appl. Surf. Sci. 292, 63–37 (2014)
J.-Ch Buhl, F. Stief, M. Fechtelkord, T.M. Gesing, U. Taphorn, C. Taake, J. Alloys Compd. 305, 93–102 (2000)
C. Baerlocher, L. McCusker, Data of zeolite structures, http://www.iza-structure.org/databases/
G. Gossner, F. Mussgnug, Z. Kristallogr. 73, 52–60 (1930)
O. Jarchow, Fortschr. Miner. 40, 55–56 (1962)
O. Jarchow, Z. Kristallogr. 122, 407–422 (1965)
H.D. Grundy, I. Hassan, Can. Miner. 20, 239–251 (1982)
I. Hassan, H.D. Grundy, Can. Miner. 29, 377–383 (1991)
I. Hassan, P.R. Buseck, Can. Miner. 30, 49–59 (1992)
M. Sirbescu, D.M. Jenkins, Am. Miner. 84, 1850–1860 (1999)
K. Hackbarth, Th..M. Gesing, M. Fechtelkord, F. Stief, J.-C. Buhl, Microporous Mesoporous Mater. 30, 347–358 (1999)
W. Eitel, Jb. Miner. II 45–61 (1922)
A.D. Edgar, B.J. Burley, Can. Miner. 7, 631–642 (1963)
A.D. Edgar, Can. Miner. 8, 53–67 (1963)
Y.I. Smolin, Y.F. Shepelev, I.K. Butikova, I.B. Kobyakov, Sov. Phys. Crystallogr. 26, 33–35 (1981)
A. Emiraliev, I.I. Yamzin, Sov. Phys. Crystallogr. 27, 27–30 (1982)
J.-C. Buhl, Thermochim. Acta. 178, 19–31 (1991)
G. Hermeler, J.-Ch Buhl, W. Hoffmann, Catal. Today. 8, 415–426 (1991)
N. Bresciani-Pahor, M. Calligaris, G. Nandin, L. Randaccio, Acta Cryst. B38, 893–895 (1982)
R. Klaska, K.-H. Klaska, O. Jarchow, Z. Kristallogr. 149, 135–137 (1979)
R.M. barrer, J.F. Cole, H. Villiger, J. Chem. Soc. A. 1523–1531 (1970)
F. Hund, Z. Anorg. Allg. Chem. 509, 153–160 (1984)
Q. Liu, H. Xu, A. Navrotsky, Microporous Mesoporous Mater. 87, 146–152 (2005)
F. Ocanto, R. Álvarez, C.U. de Navarro, A. Lieb, C.F. Linares, Microporous Mesoporous Mater. 116, 318–322 (2008)
N. Bresciani Pahor, M. Calligaris, L. Randaccio, Acta Crystallogr. B38, 893–895 (1982)
K. Latham, C.D. Williams, C.V.A. Duke, Zeolites. 17, 513–516 (1996)
G.-G. Lindner, K. Hoffmann, K. Witke, D. Reinen, C. Heinemann, W. Koch, J. Solid State Chem. 126, 50–54 (1996)
E. Gamiz, J. Linares, R. Delgado, Appl. Clay Sci. 6, 359–368 (1992)
R. Bolger, Ind. Min. 52–63 (1995)
F.H. Lin, Y.H. Lee, C.H. Jian, J.M. Wong, M.J. Shieh, C.Y. Wang, Biomaterials. 23, 1981–1987 (2002)
M.I. Carretero, Appl. Clay Sci. 21, 155–163 (2002)
A. López-Galindo, C. Viseras, P. Cerezo, Appl. Clay Sci. 36, 51–63 (2007)
J.-H. Choy, S.-J. Choi, J.-M. Oh, T. Park, Appl. Clay Sci. 36, 122–132 (2007)
A. Abdel-Motelib, Z.A. Kader, Y.A. Ragab, M. Mosalamy, Appl. Clay Sci. 52, 140–144 (2011)
I. Valentina, M. Eleonora, S. Francesca, R. Marcello, B. Alessia, T. Eleonora, M. Paola, E. Leo, Clays Clay Miner. 64(6), 719–731 (2016)
Advanced integrated, X-ray powder diffraction suite. Rigaku J. 28(1), 29–30 (2012)
E.M. Flanigen, H. Kathami, H.A. Szymanski, Adv. Chem. Ser. Mol Sieve Zeolites I 101, 201–229 (1971)
H. Shao, T.J. Pinnavaia, Microporous Mesoporous Mater. 133, 10–17 (2010)
M.C. Barnes, J. Addai-Mensah, A.R. Gerson, Colloid Surf. A 157, 101–116 (1999)
M.C. Barnes, J. Addai-Mensah, A.R. Gerson, Microporous Mesoporous Mater. 31, 287–302 (1999)
C.F. Linares, S. Sánchez, C.U. de Navarro, K. Rodríguez, M.R. Goldwasser, Microporous Mesoporous Mater. 77, 215–221 (2005)
Acknowledgements
This research was supported by the Basic Research Project (Grant 18-3214) of the Korea Institute of Geoscience and Mineral Resources (KIGAM) funded by the Ministry of Science, ICT, and Future Planning of Korea.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Seo, S.M., Kim, D., Kim, D. et al. A simple synthesis of nitrate cancrinite from natural bentonite. J Porous Mater 25, 1561–1565 (2018). https://doi.org/10.1007/s10934-018-0569-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-018-0569-4