Abstract
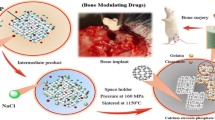
Bio-composite scaffolds were fabricated by impregnating 10, 20, 30, 40 and 50% ZrO2 content with the β-TCP matrix to heal load bearing large size bone defects. The composite scaffolds were characterized by X-ray diffraction, Fourier transform infrared spectroscopy, scanning electron microscopy and mechanical testing. The in vitro degradation of scaffolds was calculated by immersing the samples in phosphate buffer saline for a period of 21 days. Biocompatibility was evaluated by XTT assay using human Osteosarcoma cell line (MG-63). Results include scaffold surface morphology, overall porosity, phase transformation, bonding, compressive strength, biodegradability and cytotoxicity with an increase in ZrO2 percentages. The conclusions proved that β-TCP scaffold with 30% ZrO2 content exhibits the best-required properties for the application in the field of bone tissue regeneration.












Similar content being viewed by others
References
T.J. Matsumoto, S. An, T. Ishimoto, T. Nakano, T. Matsumoto, S. Imazato, Zirconia-hydroxyapatite composite material with microporous structure. Dent. Mater. 27, 205–212 (2011)
S. An, T. Matsumoto, H. Miyajima, A. Nakahira, K. Kim, S. Imazoto, Porous zirconia/hydroxyapatite scaffolds for bone reconstruction. Dent. Mater. 28, 1221–1231 (2012)
D.C. Dunand, Processing of titanium foams. Adv. Eng. Mater. 6, 369–376 (2004)
N. Kotobuki, K. Ioku, D. Kawagoe, H. Fujimori, S. Goto, H. Ohgushi, Observation of osteogenic differentiation cascade of living mesenchymal stem cells on transparent hydroxyapatite ceramics. Biomaterials 26, 779–785 (2005)
S.J. Kalita, S. Bose, H.L. Hosick, A. Bandyopadhyay, Development of controlled porosity polymer–ceramic composite scaffolds via fused deposition modeling. Mater. Sci. Eng. C 23, 611–620 (2003)
D.W. Hutmacher, M. Sittinger, M.V. Risbud, Scaffold-based tissue engineering: rationale for computer-aided design and solid free-form fabrication systems. Trends Biotechnol 22(7), 354–362 (2004)
A. Alizadeh, F. Moztarzadeh, S.N. Ostad, M. Azami, B. Geramizadeh, G. Hatam, D. Bizari, S.M. Tavangar, M. Vasei, J. Ai, Synthesis of calcium phosphate-zirconia scaffold and human endometrial adult stem cells for bone tissue engineering. Artif. Cells Nanomed. Biotechnol. 44(1), 66–73 (2014)
K.R. Mohamed, A.M. Mohamed, H.H. Beherei, Synthesis and in vitro behavior of β-TCP zirconia/polymeric biocomposites for bio-applications. J. Genet. Eng. Biotechnol. 9, 111–119 (2011)
L. Mosekilde, L. Mosekilde, Normal vertebral body size and compressive strength: relations to age and to vertebral and iliac trabecular bone compressive strength. Bone 7, 207–212 (1986)
Y. Ichikawa, Y. Akagawa, H. Nikai, H. Tsuru, Tissue compatibility and stability of a new zirconia ceramic in vivo. J. Prosthet. Dent. 68(2), 322–326 (1992)
L. Rimondini, L. Cerroni, A. Carrassi, P. Torricelli, Bacterial colonization of zirconia ceramic surfaces: an in vitro and in vivo study. Int. J. Oral Maxillofac. Implants 17(6), 793–798 (2002)
B. Stadlinger, M. Hennig, E. Kuhlisch, R. Mai, Comparison of zirconia and titanium implants after a short healing period. A pilot study in minipigs. Int. J. Oral Maxillofac. Surg. 39(6), 585–592 (2010)
Y. Akagawa, Y. Ichikawa, H. Nikai, H. Tsuru, Interface histology of unloaded and early loaded partially stabilized zirconia endosseous implant in initial bone healing. J. Prosthet. Dent. 69(6), 599–604 (1993)
R. Depprich, H. Zipprich, C. Naujoks, H. Wiesmann, S. Kiattavorncharoen, H. Lauer, U. Meyer, N. Kubler, J. Handschel, (2008), Osseointegration of zirconia implants compared with titanium: an in vivo study. Head Face Med. 4(1), 1
M. Gahlert, S. Röhling, M. Wieland, C.M. Sprecher, H. Kniha, S. Milz, Osseointegration of zirconia and titanium dental implants: a histological and histomorphometrical study in the maxilla of pigs. Clin. Oral Implants Res. 20(11), 1247–1253 (2009)
O. Hoffmann, N. Angelov, G.-G. Zafiropoulos, S. Andreana, Osseointegration of zirconia implants with different surface characteristics: an evaluation in rabbits. Int. J. Oral Maxillofac. Implants 27(2), 352–358 (2012)
P.F. Manicone, P.R. Iommetti, L. Raffaelli, An overview of zirconia ceramics: basic properties and clinical applications. J. Dent. 35(11), 819–826 (2007)
U. Meyer, M. Buhner, A. Büchter, B. Kruse-Lösler, T. Stamm, H.P. Wiesmann, Fast element mapping of titanium wear around implants of different surface structures. Clin. Oral Implants Res. 17(2), 206–211 (2006)
H. Tiainen, G. Eder, O. Nilsen, H.J. Haugen, Effect of ZrO2 addition on the mechanical properties of porous TiO2 bone scaffolds. Mater. Sci. Eng. C 32(6), 1386–1393 (2012)
G. Levita, B. Cioni, G. Gallone, A. Lazzeri, Synthesis of bioactive hydroxyapatite-zirconia toughened composites for bone replacement. Adv. Sci. Technol. 57, 31–36 (2008)
H.-W. Kim, S.-Y. Shin, H.-E. Kim, Y.-M. Lee, C.-P. Chung, H.-H. Lee, I.-C. Rhyu, Bone formation on the apatite-coated zirconia porous scaffolds within a rabbit calvarial defect. J. Biomater. Appl. 22(6), 485–504 (2008)
Z. Evis, M. Usta, I. Kutbay, Improvement in sinterability and phase stability of hydroxyapatite and partially stabilized zirconia composites. J. Eur. Ceram. Soc. 29(4), 621–628 (2009)
J. Malmstrom, E. Adolfsson, L. Emanuelsson, P. Thomsen, Bone in growth in zirconia and hydroxyapatite scaffolds with identical macroporosity. J. Mater. Sci. Mater. Med. 19(9), 2983–2992 (2007)
J.-Z. Yang, R. Sultana, X.-Z. Hu, P. Ichim, Novel layered hydroxyapatite/tri-calcium phosphate-zirconia scaffold composite with high bending strength for load-bearing bone implant application. Int. J. Appl. Ceram. Technol. 1(1), 22–30 (2013)
I. Zein, D.W. Hutmacher, K.C. Tan, S.H. Teoh, Fused deposition modeling of novel scaffold architectures for tissue engineering applications. Biomaterials 23, 1169–1185 (2001)
Z. Chen, D. Li, B. Lu, Y. Tang, M. Sun, S. Xu, Fabrication of osteo-structure analogous scaffolds via fused deposition modeling. Scr. Mater. 52, 157–161 (2005)
S.K. Sarkar, B.T. Lee, Hard tissue regeneration using bone substitutes: an update on innovations in materials. Korean J. Intern. Med. 30, 279–293 (2015)
D.-W. Jang, Y.-H. Kim, B.-T. Lee, Microstructure control of TCP/TCP-(t-ZrO2/t-ZrO2 composites for artificial cortical bone. Mater. Sci. Eng. C 31(8), 1660–1666 (2011)
D. Mondal, S. So-Ra, S.K. Sarkar, Y.K. Min, H.M. Yang, B.T. Lee, Fabrication of multilayer ZrO2-biphasic calcium phosphate-poly-caprolactone unidirectional channeled scaffold for bone tissue formation. J. Biomater. Appl. 28(3), 462–472 (2012)
R. Landers, A. Pfister, U. Hubner, H. John, R. Schmelzeisen, R. Mulhaupt, Fabrication of soft-tissue engineering scaffolds by means of rapid prototyping techniques. J. Mater. Sci. 37, 3107–3116 (2002)
P. Sapkal, A. Kuthe, R. Kashyap, A. Nayak, S. Kuthe, A. Kawle, Rapid prototyping assisted fabrication of patient-specific b-tricalcium phosphate scaffolds for bone tissue regeneration. J. Porous Mater. 23, 927 (2016)
L. Liulan, H. Xianxu, H. Qingxi, F. Minglun, Fabrication of tissue engineering scaffold via rapid prototyping machine. In: International Technology and Innovation Conference, 1280–1285, (2006)
Q. Wang, Q. Wang, C. Wan, The effect of porosity on the structure and properties of calcium polyphosphate bioceramics. Ceram.-Silik. 55(1), 43–48 (2010)
Z. Xiong, Y. Yan, S. Wang, R. Zhang, C. Zhang, Fabrication of porous scaffolds for bone tissue engineering via low-temperature deposition. Scr. Mater. 46, 771–776 (2002)
T. Serra, J.A. Planell, M. Navarro, High-resolution PLA-based composite scaffolds via 3-D printing technology. Acta Biomater. 9, 5521–5530 (2013)
N. Johari, M.H. Fathe, M.A. Golozar, E. Erfani, A. Samadikuchaksaraei, Poly(e-caprolactone)/nano fluoridated hydroxyapatite scaffolds for bone tissue engineering: in vitro degradation and biocompatibility study. J. Mater. Sci. 23, 763–770 (2012)
F.L.C. Santos, A.P. Silva, L. Lopes, I. Pires, J.I. Correia, Design and production of sintered b-tricalcium phosphate 3D scaffolds for bone tissue regeneration. Mater. Sci. Eng. C 32, 1293–1298 (2012)
Z. Li, X. Chen, N. Zhao, H. Dong, Y. Li, C. Lin, Stiff macroporous bioactive glass-ceramic scaffold: fabrication by rapid prototyping template, characterization and in vitro bioactivity. Mater. Chem. Phys. 141, 76–80 (2013)
F. Lin, C. Yan, W. Zheng, W. Fan, C. Adam, A. Oloyede, Preparation of mesoporous bioglass coated zirconia scaffold for bone tissue engineering. Adv. Mater. Res. 365, 209–215 (2012)
P. Sapkal, A. Kuthe, CAD-based approach for patient specific scaffold for bone tissue engineering, Trends Biomater. Artif. Organs 29(2), 301–305 (2015)
K. Prabhakaran, S. Kannan, S. Rajeswari, Development and characterization of zirconia and hydroxyapatite composites for orthopedic applications. Trends Biomater. Artif. Organs 18(2), 114–116 (2005)
J.P. Li, J.R. Wijn, C.A.V. Blitterswijk, K.D. Groot, Porous Ti6Al4 V scaffold directly fabricating by rapid prototyping: preparation and in vitro experiment. Biomaterials 27, 1223–1235 (2006)
S. Pattnaik, S. Nethala, A. Tripathi, S. Saravanan, A. Moorthi, N. Selvamurugan, Chitosan scaffolds containing silicon dioxide and zirconia nanoparticles for bone tissue engineering. Int. J. Biol. Macromol. 49(5), 1167–1172 (2011)
T.A. Einhorn, The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res. 355, S7–S21 (1998)
Financial disclosure
This research was not supported by any funding organization.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sapkal, P.S., Kuthe, A.M., Kashyap, R.S. et al. Indirect casting of patient-specific tricalcium phosphate zirconia scaffolds for bone tissue regeneration using rapid prototyping methodology. J Porous Mater 24, 1013–1023 (2017). https://doi.org/10.1007/s10934-016-0341-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-016-0341-6