Abstract
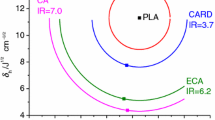
We report the use of novel biobased plasticizers prepared starting from citric acid (CA) in polylactic acid (PLA). Citric acid based plasticizers are well-known softeners for PLA, with citrate esters as the most commonly used class. However, citrate esters are known to leach out of the plastic material over time. This problem is currently addressed by acetylating the tertiary hydroxyl group of citric acid via complex and environmentally polluting processes. An alternative strategy consists in the reductive removal of the tertiary hydroxyl group, resulting in propane-1,2,3-tricarboxylic acid. Derivatizing this compound leads to novel plasticizers which have not been tested in PLA yet. Here, different esters of propane-1,2,3-tricarboxylic acid were synthesized and blended in PLA. Their influence on the thermal (Tg and Tm) and the mechanical properties (Young’s modulus, stress and strain) of PLA along with their migration out of the material were compared to those of commercially available citric acid based plasticizers. Our results show that similar or better results were obtained with these new PLA plasticizers.




Similar content being viewed by others
References
Plastic - the facts 2021 (2022) Plastic Europe, Brussels. https://plasticseurope.org. Accessed April 2022
A cicrcular economy for plastics (2020) Publications office of the EU, Luxembourg. https://op.europa.eu/. Accessed august, 2022
Business insights - Global trends (2021) Plastic Industry Association, Washington DC. https://www.plasticsindustry.org. Accessed August, 2022
Dusselier M, Van Wouwe P, Dewaele A, Makshina E, Sels BF (2013) Lactic acid as a platform chemical in the biobased economy: the role of chemocatalysis. Energy Environ Sci 6(5):1415–1442. https://doi.org/10.1039/c3ee00069
Vink ETH, Rábago KR, Glassner DA, Gruber PR (2003) Applications of life cycle assessment to NatureWorksTM polylactide (PLA) production. Polym Degrad Stab 80(3):403–419. https://doi.org/10.1016/S0141-3910(02)00372-5
Tuck CO, Pérez E, Horváth IT, Sheldon RA, Poliakoff M (2012) Valorization of biomass: Deriving more value from waste (Science (695)). Science 338(6107):604. https://doi.org/10.1126/science.338.6107.604-b
Vert M (1989) Bioresorbable polymers for temporary therapeutic applications. Macromol Mater Eng 166(1):155–168. https://doi.org/10.1002/apmc.1989.051660111
Greco A, Gennaro R, Timo A, Bonfantini F, Maffezzoli A (2013) A comparative study between bio-composites obtained with opuntia ficus indica cladodes and flax fibers. J Polym Environ 21(4):910–916. https://doi.org/10.1007/s10924-013-0595-x
Södergård A, Solt M (2007) Properties of polylactic acid fiber based polymers and their correlation with composition. Proceedings of 2007 International Conference on Advanced Fibers and Polymer Materials (ICAFPM), 1:8–11
Gupta AP, Kumar V (2007) New emerging trends in synthetic biodegradable polymers - Polylactide: a critique. Eur Polym J 43(10):4053–4074. https://doi.org/10.1016/j.eurpolymj.2007.06.045
Auras RA, Lim LT, Selke SEM, Tsuji H (2010) Poly(Lactic Acid): synthesis, Structures, Properties, Processing And Applications. Wiley, New York
Gruber P, Henton DE, Starr J (2008) Biorefineries - Industrial Processes and Products. Wiley, Weinheim, pp 381–407
Lunt J (1998) Large-scale production, properties and commercial applications of poly lactic acid polymers. Polym Degrad Stab 59(1–3):145–152. https://doi.org/10.1016/s0141-3910(97)00148-1
Brant DA, Tonelli AE, Flory PJ (1969) The configurational statistics of random poly (lactic acid) chains II Theory. Macromolecules 2(3):228–235. https://doi.org/10.1021/ma60009a003
Joziasse CAP, Veenstra H, Grijpma DW, Pennings AJ (1996) On the chain stiffness of poly(lactide)s. Macromol Chem Phys 197(7):2219–2229. https://doi.org/10.1002/macp.1996.021970713
Ge H, Yang F, Hao Y, Wu G, Zhang H, Dong L (2013) Thermal, mechanical, and rheological properties of plasticized poly(L -lactic acid). J Appl Polym Sci 127(4):2832–2839. https://doi.org/10.1002/app.37620
Sears JK, Darby JR (1982) The Technology of Plasticizers. Wiley, New York
Mascia L, Xanthos M (1992) An overview of additives and modifiers for polymer blends: facts, deductions, and uncertainties. Adv Polym Technol 11(4):237–248. https://doi.org/10.1002/adv.1992.060110402
Yu RL, Zhang LS, Feng YH, Zhang RY, Zhu J (2014) Improvement in toughness of polylactide by melt blending with bio-based poly(ester)urethane. Chin J polym sci (English Edition) 32(8):1099–1110. https://doi.org/10.1007/s10118-014-1487-9
Cerdan K, Brancart J, Roels E, Vanderborght B, Van Puyvelde P (2022) Humins blending in thermoreversible Diels-Alder networks for stiffness tuning and enhanced healing performance for soft robotics. Polymers 14(9):1657. https://doi.org/10.3390/polym14091657
Eguiburu JL, Iruin JJ, Fernandez-Berridi MJ, San Román J (1998) Blends of amorphous and crystalline polylactides with poly(methyl methacrylate) and poly(methyl acrylate): a miscibility study. Polymer 39(26):6891–6897. https://doi.org/10.1016/S0032-3861(98)00182-7
Flory PJ (1953) Principles of Polymer Chemistry. Cornell University Press, New York
Sheth M, Kumar RA, Davé V, Gross RA, McCarthy SP (2008) Biodegradable polymer blends of poly (lactic acid) and Poly ( ethylene glycol ). J Appl Polym Sci 66(8):1495–1505
Ruan G, Feng SS (2003) Preparation and characterization of poly(lactic acid)-poly(ethylene glycol)-poly(lactic acid) (PLA-PEG-PLA) microspheres for controlled release of paclitaxel. Biomaterials 24(27):5037–5044. https://doi.org/10.1016/S0142-9612(03)00419-8
Younes H, Cohn D (1988) Phase separation in poly(ethylene glycol)/poly(lactic acid) blends. Eur Polym J 24(8):765–773. https://doi.org/10.1016/0014-3057(88)90013-4
Jacobsen S, Fritz HG (1999) Plasticizing polylactide—the effect of different plasticizers on the mechanical properties. Polym Eng Sci 39(7):1303–1310. https://doi.org/10.1002/pen.11517
Baiardo M, Frisoni G, Scandola M, Rimelen M, Lips D, Ruffieux K, Wintermantel E (2003) Thermal and mechanical properties of plasticized poly(L-lactic acid). J Appl Polym Sci 90(7):1731–1738. https://doi.org/10.1002/app.12549
Kowalczyk M, Pluta M, Piorkowska E, Krasnikova N (2012) Plasticization of polylactide with block copolymers of ethylene glycol and propylene glycol. J Appl Polym Sci 125:4292–4301. https://doi.org/10.1002/app.36563
Chieng BW, Ibrahim NA, Yunus WMZW, Hussein MZ (2013) Plasticized poly(lactic acid) with low molecular weight poly(ethylene glycol): mechanical, thermal, and morphology properties. J Appl Polym Sci 130(6):4576–4580. https://doi.org/10.1002/app.39742
Piorkowska E, Kulinski Z, Galeski A, Masirek R (2006) Plasticization of semicrystalline poly(l-lactide) with poly(propylene glycol). Polymer 47(20):7178–7188. https://doi.org/10.1016/j.polymer.2006.03.115
Bocqué M, Voirin C, Lapinte V, Caillol S, Robin JJ (2016) Petro-based and bio-based plasticizers: chemical structures to plasticizing properties. J Polym Sci Part A: Polym Chem 54(1):11–33. https://doi.org/10.1002/pola.27917
Sinclair RG (1996) The case for polylactic acid as a commodity packaging plastic. J Polym Sci, Part A: Polym Chem A33:585–597. https://doi.org/10.1080/10601329608010880
Burgos N, Martino VP, Jiménez A (2013) Characterization and ageing study of poly(lactic acid) films plasticized with oligomeric lactic acid. Polym Degrad Stab 98(2):651–658. https://doi.org/10.1016/j.polymdegradstab.2012.11.009
Chieng BW, Ibrahim NA, Then YY, Loo YY (2014) Epoxidized vegetable oils plasticized poly(lactic acid) biocomposites: mechanical, thermal and morphology properties. Molecules 19(10):16024–16038. https://doi.org/10.3390/molecules191016024
Carbonell-Verdu A, Samper MD, Garcia-Garcia D, Sanchez-Nacher L, Balart R (2017) Plasticization effect of epoxidized cottonseed oil (ECSO) on poly(lactic acid). Ind Crops Prod 104:278–286. https://doi.org/10.1016/j.indcrop.2017.04.050
Maiza M, Benaniba MT, Quintard G, Massardier-Nageotte V (2015) Biobased additive plasticizing Polylactic acid (PLA). Polimeros 25(6):581–590. https://doi.org/10.1590/0104-1428.1986
Harte I, Birkinshaw C, Jones E, Kennedy J, Debarra E (2013) The effect of citrate ester plasticizers on the thermal and mechanical properties of poly(DL -lactide). J Appl Polym Sci 127(3):1997–2003. https://doi.org/10.1002/app.37600
Labrecque LV, Kumar RA, Davé V, Gross RA, Mccarthy SP (1997) Citrate esters as plasticizers for poly(lactic acid). J Appl Polym Sci 66(8):1507–1513
Polymer additives – Comprehensive guide on plasticizers (2022) Special Chem, Paris. https://polymer-additives.specialchem.com. Accessed August, 2022
Mores S, de Vandenberghe LP S, Magalhães Júnior AI, de Carvalho JC, de Mello AFM, Pandey A, Soccol CR (2021) Citric acid bioproduction and downstream processing: Status, opportunities, and challenges. Bioresour Technol 320:124426. https://doi.org/10.1016/j.biortech.2020.124426
Immergut EH, Mark HF (1965) Advances in chemistry, plasticization and plasticizer processes. Am Chem Soc 48:1–26. https://doi.org/10.1021/ba-1965-0048.ch001
Ljungberg N, Wesslén B (2005) Preparation and properties of plasticized poly(lactic acid) films. Biomacromol 6(3):1789–1796. https://doi.org/10.1021/bm050098f
Nara K, Nishiyama K, Natsugari H, Takeshita A, Takahashi H (2009) Leaching of the plasticizer, acetyl tributyl citrate: (ATBC) from plastic kitchen wrap. J Health Sci 55(2):281–284. https://doi.org/10.1248/jhs.55.281
Li H, Huneault MA (2007) Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 48(23):6855–6866. https://doi.org/10.1016/j.polymer.2007.09.020
Liu J, Yuan R, Sang Q, Dang L, Gao L, Xu B, Xu S (2023) Effect of acetylated citrate plasticizer on mechanical properties of poly(vinyl chloride). Mater Chem Phys 295:127068. https://doi.org/10.1016/j.matchemphys.2022.127068
Ting S (2007) Synthetic method of high-purity acetyl tributyl citrate (ATBC). Patent CN 101353305B
Song BL, Zhang G, Wang YZ, Yanli WJ (2008) Process for producing acet-tributyl citrate. Patent CN 101402571A
Kia G, Yang Z, Pengwei Z, Wei H, Xiaolin L (2011) A process for synthesizing acetyl citrate. Patent, CN 102351696B
Jianzhong L, Yuepeng Z, Hiulai L (2012) Integrated production technique of acetyl tributyl citrate (ATBC). Patent, CN 102633640B
Xingjian C (2017) An efficient synthesis method for acetyl tributyl citrate. Patent, CN 106928065A
Day JF (2004) Acylated esters. Patent, US 20060094894A1
Bergrath K, Klein T, Bohnen H (2002) Method for producing ester of citric acid. Patent US 2002(0111508):A1
Frappier EP, Kennedy SW (2007) Tricarboxylic acid ester plasticizer and methods of making. Patent US 2007(0072988):A1
Verduyckt J, De Vos DE (2017) Highly selective one-step dehydration, decarboxylation and hydrogenation of citric acid to methylsuccinic acid. Chem Sci 8(4):2616–2620. https://doi.org/10.1039/c6sc04541c
Verduyckt J, Geers A, Claes B, Eyley S, Van Goethem C, Stassen I, Smolders S, Ameloot R, Vankelecom I, Thielemans W, De Vos DE (2017) Stabilising Ni catalysts for the dehydration-decarboxylation-hydrogenation of citric acid to methylsuccinic acid. Green Chem 19(19):4642–4650. https://doi.org/10.1039/c7gc01773a
Li Z, Wen X, Liu H (2022) Efficient conversion of bio-renewable citric acid to high-value carboxylic acids on stable solid catalysts. Green Chem 24(4):1650–1658. https://doi.org/10.1039/d1gc04497d
Stuyck W, Verduyckt J, Krajnc A, Mali G, De Vos DE (2020) Selective defunctionalization of citric acid to tricarballylic acid as a precursor for the production of high-value plasticizers. Green Chem 22(22):7812–7822. https://doi.org/10.1039/d0gc02298e
Stuyck W, Bugaev AL, Nelis T, de Oliveira-Silva R, Smolders S, Usoltsev OA, Arenas Esteban D, Bals S, Sakellariou D, De Vos DE (2022) Sustainable formation of tricarballylic acid from citric acid over highly stable Pd/Nb2O5·nH2O catalysts. J Catal 408:88–97. https://doi.org/10.1016/j.jcat.2022.02.013
De Bruyne A, Stuyck W, Deleu W, Leinders J, Marquez C, Janssens K, Sakellariou D, Ghillebert R, De Vos ED (2023) Efficient two-step production of biobased plasticizers: dehydration-hydrogenation of citric acid followed by Fischer esterification. Green chem 25:3896–3908. https://doi.org/10.1039/D2GC04678D
Magne FC, Mod RR (1953) Plasticizers from aconitic and tricarballylic acids. Ind Eng Chem 45(7):1546–1547. https://doi.org/10.1021/ie50523a049
Reid RJ, Smith Junior WM, Werner BH (1957) Plastication Of Vinidylene Resins With Tricarballylates. Patent US 2802802
Stuart A, McCallum MM, Fan D, LeCaptain DJ, Lee CY, Mohanty DK (2010) Poly(vinyl chloride) plasticized with succinate esters: synthesis and characterization. Polym Bull 65:589–598. https://doi.org/10.1007/s00289-010-0271-4
Erythropel HC, Dodd P, Leask RL, Maric M, Cooper DG (2013) Designing green plasticizers: Influence of alkyl chain length on biodegradation and plasticization properties of succinate based plasticizers. Chemosphere 91:358–365. https://doi.org/10.1016/j.chemosphere.2012.11.061
Erythropel HC, Shipley S, Börmann A, Nicell JA, Maric M, Leask RL (2016) Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer 89:18–27. https://doi.org/10.1016/j.polymer.2016.02.031
Denayer M, Vekeman J, Tielens F, De Proft F (2021) Towards a predictive model for polymer solubility using the noncovalent interaction index: polyethylene as a case study. Phys Chem Chem Phys 23(44):25374–25387. https://doi.org/10.1039/D1CP04346C
Abraham MJ, Murtola T, Schulz R, Páll S, Smith JC, Hess B, Lindahl E (2015) GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1(2):19–25. https://doi.org/10.1016/j.softx.2015.06.001
Evans DJ, Holian BL (1985) The nose-Hoover thermostat. J Chem Phys 83:4069–4074. https://doi.org/10.1063/1.449071
Parrinello M, Rahman A (1981) Polymorphic transitions in single crystals: a new molecular dynamics method. J Appl Phys 52(12):7182–7190. https://doi.org/10.1063/1.328693
Jorgensen WL, Tirado-Rives J (1988) The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J Am Chem Soc 110(6):1657–1666. https://doi.org/10.1021/ja00214a001
Hockney RW, Goel SP, Eastwood JW (1974) Quiet high-resolution computer models of a plasma. J Comput Phys 14(2):148–158. https://doi.org/10.1021/ja00214a001
Essmann U, Perera L, Berkowitz ML, Darden T, Lee H, Pedersen LG (1995) A smooth particle mesh Ewald method. J Chem Phys 103(19):8577–8593. https://doi.org/10.1063/1.470117
Berendsen HJC, Postma JPM, van Gunsteren WF, DiNola A, Haak JR (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81(8):3684–3690. https://doi.org/10.1063/1.448118
Wypych G (2012) Handbook of Plasticizers. Elsevier, Toronto
Daniels PH (2009) A brief overview of theories of PVC plasticization and methods used to evaluate PVC-plasticizer interaction. J Vinyl Addit Technol 15(4):219–223. https://doi.org/10.1002/vnl.20211
Siepmann J, Paeratakul O, Bodmeier R (1998) Modeling plasticizer uptake in aqueous polymer dispersions. Int J Pharm 165(2):191–200. https://doi.org/10.1016/S0378-5173(97)00390-6
Wang Y, Qin Y, Zhang Y, Yuan M, Li H, Yuan M (2014) Effects of N-octyl lactate as plasticizer on the thermal and functional properties of extruded PLA-based films. Int J Biol Macromol 67:58–63. https://doi.org/10.1016/j.ijbiomac.2014.02.048
Herrera N, Mathew AP, Oksman K (2015) Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: Mechanical, thermal and optical properties. Compos Sci Technol 106:149–155. https://doi.org/10.1016/j.compscitech.2014.11.012
Barbosa JL, Perin GB, Felisberti MI (2021) Plasticization of Poly(3-hydroxybutyrate- co-3-hydroxyvalerate) with an oligomeric polyester: miscibility and effect of the microstructure and plasticizer distribution on thermal and mechanical properties. ACS Omega 6(4):3278–3290. https://doi.org/10.1021/acsomega.0c05765
Bose S, Roy M, Bandyopadhyay A (2012) Recent advances in bone tissue engineering scaffolds. Trends Biotechnol 30(10):546–554. https://doi.org/10.1016/j.tibtech.2012.07.005
Acknowledgements
This project has received funding from VLAIO (HBC.2019.2387), in a collaborative project between Citribel and KULeuven. Wouter Stuyck is grateful to the FWO for his SB PhD fellowship (1SC1519N). F.D.P., D.D.V. and M.D. acknowledge the Research Foundation—Flanders (FWO) for financial support through SBO grant S001819N. The computational resources and services used in this work were provided by the VSC (Flemish Supercomputing Center), funded by the Research Foundation—Flanders (FWO) and the Flemish Government. J.V. acknowledges the Research Foundation—Flanders (FWO) for a junior postdoctoral mandate (Project No. 12E6423N). The authors would like the thank A. Vananroye, C. Poisson and V. Jeroen for performing training in using the various tools used in this work.
Funding
Agentschap Innoveren en Ondernemen, HBC.2019.2387, Fonds Wetenschappelijk Onderzoek, S001819N, 12E6423N, S001819N, 1SC1519N.
Author information
Authors and Affiliations
Contributions
A.D.B wrote the main manuscript text and obtained the larger part of the results. K.C provided results regarding the mechanical properties (Table 5 + suppl. info.) and reviewed the manuscript. G.R. provided/helped with TGA measurements (Table 6 + suppl. info.). M.D., J.V. and F.P provided results regarding Computational methods (Figure 3). W.S. supervised research and reviewed the manuscript. J.L. provided/helped with DSC results. D.D.V and P.V.P provided intellectual input, techniques used in this work (TGA, DSC, NMR, etc.), supervised research and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
De Bruyne, A., Gómez, K.C., O’Rourke, G. et al. New Tricarboxylate Plasticizers for Use in Polylactic Acid: Synthesis, Thermal Behavior, Mechanical Properties and Durability. J Polym Environ (2024). https://doi.org/10.1007/s10924-024-03254-0
Accepted:
Published:
DOI: https://doi.org/10.1007/s10924-024-03254-0