Abstract
One of the major environmental issues that modern civilizations are currently dealing with is the growing amount of plastic waste. Because of how they affect all forms of life, this waste is seen as a severe worldwide issue. Current methods for plastic waste disposal do not offer definitive solutions and often lead to the production of microplastics or secondary pollution. In recent years there has been a growing interest by the scientific community in the degradation of plastics by biological means, in particular the possibilities of using insects as a potential solution to the accumulation of this type of waste have been investigated. Among these, one of the most promising is undoubtedly the lepidopteran Galleria mellonella, which synthesizes the first ever discovered polyethylene degrading enzymes. In this review we propose an overview of plastic polymers production and common degradation methodologies, and analyses the current state of the art about the degradation carried out by this insect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction—Plastic Industry and Waste Production
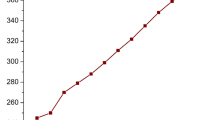
Few industries have grown as much in the past 70 years as the plastic business, both in terms of output tons (as can be seen from the graph in Fig. 1) and usage in almost every aspect of our daily lives. This is owing to the exceptional qualities of plastics that have developed over time, such as their low cost, stability, and resilience due both to their polymeric features and to the presence of additives [1, 2]. Since the beginning of the twenty-first century, the worldwide plastic manufacturing has nearly doubled. Population growth, rising purchasing power and growing demand for plastic products all point to continued growth in plastic production. Indeed, the global plastics market has been estimated at $580 and $593 billion in 2020 and 2021, respectively, but it is expected to increase significantly in the following decade, achieving a value of more than 810 billion U.S. dollars by 2030 [3]. The European plastic industry, which includes plastics manufactures, recycles and converters, and machinery manufactures, is made by more than 52 000 companies and employs about 1.5 million people, ranking 8th in Europe in industrial value-added contribution with a revenue of approximately 405 billion euros in 2021 [4].
Annual production of plastics worldwide from 1950 to 2021 [5].
According to Plastic Europe, global plastics production climbed to 390.7 million tonnes in 2021 after experiencing a halt in 2020 as a result of the Covid-19 epidemic. Of that, 352.3 million tonnes (90.20%) were fossil-based plastic (with polyethylene -PE- and polypropylene -PP- representing the most produced type of plastic, as can be seen from the graph in Fig. 2), while only 32.5 million tonnes (8.30%) and 5.9 million tonnes (1.50%) were post-consumer recycled plastic and bio-based plastics respectively. In Europe the manufacturing of plastics was made up of 10.10% post-consumer recycled plastic and 2.30% bio-based/bio-attributed plastic; however still 87.60% (approximately 50.1 million tonnes) were produced from fossil-based feedstock [4].
Global plastic polymers production by type in 2021, according to Plastics Europe [4].
The two biggest global plastics markets in 2021 were packaging (44%) and building & construction (18%) applications, with PE (low density to high density), PP and polystyrene (PS) accounting for more than half (51.50%) of the total plastic production [4]. Plastic can have a very short useful life, which is one of its traits, notably in the packaging industry. Plastic is often only used once before being discarded; this is clearly an intrinsic problem of the plastic industry since years are required for the degradation of the plastic in the environment [6]. Plastics are not biodegradable and, as a result, they build up in landfills or the environment rather than decomposing [7]. With an estimated 4 to 12 million metric tons of plastic garbage produced on land entering the marine environment in 2010 alone [8], plastic debris has been discovered in all major ocean basins [7]. There are also more and more reports of freshwater systems and terrestrial habitat contamination [9,10,11,12,13]. The environment today contains so much plastic waste that it has been recommended as a geological sign of the hypothesized Anthropocene period [14, 15]. Bioplastics, which, as defined by European Bioplastics Society, are either biobased plastics, biodegradable plastics, or combine both characteristics [16], are not the solution to this problem, since they are not necessarily biodegradable. In fact, the popularity of bioplastics was initially underpinned by the idea that these polymers were 100% biodegradable, compostable and environmentally friendly [17]. This idea could be misleading since not all bioplastics are compostable and many of them are, in fact, non-biodegradable at all. Indeed, approximately 48% of the bioplastics produced in 2022 were non-biodegradable [18]. Example of non-biodegradable plastics are bio-PE, bio-PP, bio-polyethylene-terephthalate (bio-PET), bio-polyytrimethylene terephthalate (bio-PTT), and bio-polyamide (bio-PA) [19].
When treating with plastic waste, incineration is usually regarded as a viable response to the problem of accumulating plastics; but due to the emission of greenhouse gases and harmful pollutants into the atmosphere, such as dioxins and dioxin-like compounds, carbon monoxide, nitrogen oxides, etc., this process also has detrimental effects on the environment [1].
Mechanical recycling and mechanical sorting are gradually taking over as the preferred technique for reusing thermoplastic waste. Unfortunately, mechanical recycling leads to lower-quality and, after multiple recycling cycles, most plastic materials suffer significant damage to their physical and chemical properties [20, 21], The range of suitable thermoplastics for secondary recycling is also restricted to those that are not sensitive to temperature changes and do not have high melt viscosities [22]. High density PE (HDPE) and PET are two of the most common forms of plastic polymers that are recycled mechanically, while less than 1% of non-biodegradable plastic waste processed by mechanical recycling is made up of other types of polymers like PP [23]. New methods for reusing plastic waste are therefore needed. One of these methodologies may be chemical recycling, which has the potential to recover monomers and other useful compounds from plastic trash. The success of chemical recycling, however, depends on the effectiveness of used catalysts and the commercial viability of proposed methods [24]. In addition, with chemolysis it is challenging to recover polymers like PE and PP that only contain C–C and C–H bonds [25]. Despite its high demand of land required, landfilling is still the preferred method for disposing of plastic trash in underdeveloped nations due to its advantages as a low-cost waste management solution [21]. Landfilling is the most used way to deal with plastic waste also in countries like the USA, where in 2018 75.60% of plastic waste ended up in landfills, while the remaining 8.70% and 15.70% were recycled or incinerated for energy production, respectively [26]. Environmental factors, such as photooxidation or heat, which occurs frequently when plastic waste is disposed of in landfills, results in the development of tiny fragments with a diameter of less than 5 mm, known as microplastics [27], which have an adverse effect on living organisms. Unfortunately, these microplastics are not only derived from the degradation of traditional fossil-based plastics, but also from bioplastics: microplastics from biodegradable polyester were noted in both freshwater and seawater [28].
Unfortunately, according to a new analysis of Organisation for Economic Cooperation and Development, the amount of plastic trash produced globally is going to nearly triple by 2060, with about half going to landfills and fewer than a fifth being recycled [29].
Plastic Abiotic and Biotic Degradation
Abiotic Degradation
Abiotic degradation can be divided mainly into photodegradation, thermal and mechanical degradation [30]. The most significant process that starts the decomposition of plastic in the environment is known as photodegradation. Plastics typically photodegrade through processes mediated by free radicals that are started by sunlight, as the main responsible are UV-B (290–315 nm) and UV-A (315–400 nm) radiations [31]. These radiations can break the C–C or C–H bonds of the polymeric chain thanks to their high energy and, by doing so, initiate the process of degradation. Chain scission, crosslinking, and secondary oxidative reactions, which occur through the production and transfer of free radicals, are part of the processes that contribute to the photodegradation of polymers [32]. The term “thermal degradation” describes the breakdown of polymers as a result of energy input from high temperatures, and generally occurs in the presence of oxygen. At high temperatures, thermo-oxidative reactions can occur with plastics. Long polymer chains may break and produce radicals when enough heat is absorbed by the polymer to break through the energy barrier [33]. Slow thermal oxidation of plastics may occur in conjunction with photodegradation. Temperature and UV light can have synergistic effects on the breakdown of plastics, and the pace of oxidative reactions also rises with temperature [34]. The expression “mechanical deterioration” refers to the way polymers break down due to environmental mechanical factors such as plastics rubbing up against rocks and sand due to wind and waves. The freezing and thawing of plastics under water conditions can also cause the mechanical breakdown of polymers [35]. Finally, pollutants including ozone (O3), sulphur dioxide (SO2) or nitrogen dioxide (NO2) in the atmosphere can either directly damage plastics or stimulate the creation of radicals through photochemical reactions, which may also cause plastics to degrade [36].
What Makes Synthetic Polymers Prone or Resistant to Degradation?
According to the presence or lack of ester or amide groups, which can be attacked by different extracellular hydrolases, synthetic plastics can be classified as hydrolysable or non-hydrolysable. Among the latter, there are the polyolefins, a class of thermoplastic materials that includes PE, PP and PS. These are created by polymerizing olefin monomer units, such as ethylene, propylene and styrene, respectively [37, 38]. Due to the energies of the C–C and C–H bonds, substantially higher than those of the C–O and C–N bonds, polyolefins are more resistant to degradation than ester-bonded polymers like PET, and represent the main problem regarding plastic waste management. Indeed, polymers like PET (or polyesters in general) are hydrolysable, hence typically more vulnerable to biodegradation [39]. Several enzymes such as lipase, cutinase, serine esterase and nitro-benzyl-esterase have the ability to hydrolyze PET [30]. Moreover, the integration of heteroatoms into the carbon chain makes hydrolysable plastics like PET more sensitive both to biotic and abiotic degradation [40]. Extracellular enzyme degradation of non-hydrolysable polyolefins like PE and PP is more challenging [30], which is unfortunate as they represent the vast majority of the plastic market.
During the manufacturing process, the addition of additives (such as stabilizers) that aim to improve the rheological, mechanical, thermal and electrical properties of the polymer produced affects the degradability of the material [21]. Hydrophobicity also typically has an impact on how efficiently polymers degrade, with the degradation rate improving as hydrophobicity decreases [21]. Another aspect that decreases the rate of degradation is the crystallinity of the polymer [41]. Finally, polymers with high MW degrade more slowly because their relative surface area is smaller [40]. Not only the properties of the polymer itself, but also those of the environment in which it is located, influence the rate of degradation of the plastic material; indeed, the rates of deterioration increase with warmth and in the presence of high levels of oxygen or water [42,43,44,45].
Biotic Degradation
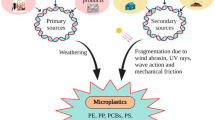
For the handling of plastic waste, an effective and environmentally beneficial strategy is currently lacking and could be represented by the biodegradation methods that need to be improved. The biodegradation is defined by IUPAC as “Breakdown of a substance catalyzed by enzymes in vivo” [46]. It is widely acknowledged that under environmental circumstances, the process of biodegradation by microorganisms is incredibly slow and it requires months or more, with the exception of amorphous PET [47]. Given the insolubility of the material in aqueous media, the high molecular weight (MW) of the polymer, and the potential release of hazardous chemicals throughout the process, microbial activity may be physically constrained by the plastic substrate [48]. The paradigm used in the biodegradation field is a consequence of the idea of biodegradation occurring in the environment by microorganisms. Therefore, the plastic biodegradation pathway, necessarily by microorganisms in the environment, is usually divided into four processes: biodeterioration, biofragmentation, microbiological absorption, and biomineralization (Fig. 3) [49]. The theory dictates that microbial enzymes are initially released into the environment and can directly interact with plastic surfaces to cause biodeterioration. Then the oligomers that form are liberated during biofragmentation through the depolymerization of plastic. Microorganisms have their cell walls and membranes penetrated by these plastic oligomers, which are degraded into monomers by intracellular enzymes. Thus, these microbes consume monomers to produce cellular biomass. Eventually, microbes totally metabolize biomass as an elementary component, biomineralizing it into carbon dioxide or methane (depending on the oxygen availability) and water [50,51,52]. Many different bacterial and fungal species have been observed attaching to and degrading petroleum-based polymers [27, 53,54,55]. The main bacterial species involved in polymer degradation are Pseudomonas aeruginosa, Pseudomonas stutzeri, Streptomyces badius, Streptomyces setonii, Rhodococcus ruber, Comamonas acidovorans, Clostridium thermocellum, and Butyrivibrio fibrisolvens; while the main fungal species are Aspergillus niger, Aspergillus flavus, Fusarium lini, Pycnoporus cinnabarinus and Monilesaurus rouxii [55]. For example, R. ruber C208, one of the most efficient bacteria, has been reported to be able to degrade non-pretreated low-density polyethylene (LDPE) and PS, with a degradation rate of 0.90% per week for LDPE [56, 57], where changes in its MW and molecular number were also noticed [58], while a weight loss of 0.80% was achieved for PS in 8 weeks [59]; although it has been reported how the degradation rate increases when coupled with a UV pretreatment [60]. Along with individual bacteria, numerous microbial consortia may break down plastic polymers and microorganisms functioning as a consortium can have higher biodegradation efficiency than individual strains, either by directly participating in the process or by removing potentially harmful degradation intermediates [61]. A consortium of five different strains of Bacillus and Pseudomonas was able to grow synergistically using PET as the only carbon source, demonstrating collaboration and the capacity to cross-feed in a nutrient-limited environment [62], then by using the entire consortium’s pangenome, the researchers were able to identify many hydrolases, oxidoreductases and dehydrogenases with potential for PET degradation [63]; additionally, the scientists claim that the entire consortium looks to have a pangenome that can break down different kinds of plastic like polyurethane (PU) [63]. Through metabolic cross-feeding or the production of metabolites that promote co-metabolic degradation, specific members of a microbial collective can also indirectly enhance biodegradation. It was demonstrated that PE can be broken down after a 126-day incubation in soil by a microbial consortium formed by Lysinibacillus xylanilyticus and A. niger, but the degradation was vastly improved by an UV pretreatment [64]; while another consortium made up of Brevibacillus sp. And Aneurinibacillus sp. isolated from waste landfills was able to produce a considerable weight loss in LDPE and HDPE strips and pellets (with an average of 51.95% and 41.90% weight loss for LDPE and HDPE, respectively) following a 140-day incubation [65]. Another consortium made up of several microorganisms (Burkholderia sp., Methylocystis sp., Methylocella sp., Methylobacter sp., Methylococcus capsulatus., Nitrosomonas sp., Nitrosomonas europaea, Nitrobacter winogradskyi, Nitrobacter hamburgensis, Pseudomonas sp. and Xanthobacter sp.) was able to partially degrade UV pretreated LDPE and HDPE after a 90 day-incubation, with the formation of several degradation products like alkanes, alkenes and alcohols [66]. However, this biodegradation procedure might take a long time, especially without an UV or thermo-oxidative pretreatment that can enhance the process [60, 64, 66,67,68]; abiotic degradation of plastics in fact is the best way in which microbes are then able to degrade polyolefins (like PE, PP and PS), which are characteristically inert and immune to microbial attack (fungi, bacteria, etc.) [69]. This paradigm associated with the four steps designated in the field of biodegradation is the reference point when it comes to the biodegradation of plastics. Even today, most studies focus almost exclusively on the role of microorganisms, such as bacteria and fungi, in the biodegradation process of polyolefins, but unfortunately there is still a lack of microbial enzymes that can catalyze this process.
Plastic Degradation by Insects
The degrading action of insects on various types of plastic material does not fit into the current definition of microorganism-mediated biodegradation divided into the four steps mentioned above, and, for this reason, we will refer to this activity as the degradation (and not biodegradation) of plastic by insects. The first time the ability of an insect to damage plastic packaging was noticed in the 1950s, when scientists found that some beetles and their larvae, belonging to Tenebrionidae, Anobiidae, and Dermestidae families, had an intriguing capacity to damage plastic wrappings [70]. At that period, insects capable of damaging plastics were seen in a negative way and finding repellent solutions or resistant materials to prevent insect penetration in packing films was the goal. On the contrary, nowadays this peculiar ability displayed by various insects is seen as a possible new and green solution to the problem of plastic waste disposal.
Since then, several insects have shown this ability, in particular in the Lepidoptera and Coleoptera order. Among them Galleria mellonella is certainly one of the most studied and promising. This modern interest in insects and plastic degradation began in 2007, when Riudavates and colleagues studied the damages produced by three insect species (the Coleoptera Rhyzopertha dominica, Sitophilus oryzae and Lasioderma serricorne) in food packaging film composed of PP, PE and polyester [71]. Although the paper was mainly focused on the detection of insect contamination in food, it has surely paved the way for the interest in plastic degradation by insects. Indeed, in 2010 Miao and Zhang [72] published a paper about Zophobas atratus (Tenebrionidae: Coleoptera) and his ability to biodegrade plastics: according to the authors, the larvae consumed LDPE, linear low-density polyethylene (LLDPE), PS, and polyvinyl chloride (PVC) [71]. They declared that the larvae ingested 2.4 g of PS per Kg of larvae per day, but the study lacked strong data on degradation for all polymers: changes in the thermal characteristics and stability of leftover PVC and PS were noted based on thermogravimetric analysis (TGA) of larval frass, but not for LDPE [72]. This study opened a niche within the scientific world.
In 2014 Yang et al. [73] noticed the ability of another insect larvae to degrade PE, Plodia interpunctella (Lepidoptera: Pyralidae). The authors were able to isolate two bacteria from the intestine of this lepidopteran, Enterobacter asburiae YT1 and Bacillus sp. YP1, and to show that these had degrading activity toward PE. However, the results of these isolations did not lead to further study in this regard.
In 2015 another Tenebrionidae, Tenebrio molitor, was investigated. Yang et al. [74, 75] demonstrated the ability of this insect’s larvae to biodegrade PS and they assumed that this process was due to the microbiome of the larvae since mealworms' capacity to depolymerize long-chain PS molecules and further mineralize the metabolites to CO2 was hampered by antibiotic suppression of gut flora [75]. They also isolated a PS degrading bacterial strain, Exiguobacterium sp. YT2, from T. molitor larval microbiome but the microorganism alone showed a much slower and inefficient PS degradation [75], suggesting a possible active role of the insects in this process.
Finally, the ability of G. mellonella larvae to degrade plastic was noticed for the first time in 2017, by Bombelli et al. [76], that found out that the larvae left in contact with a commercial shopping bag produced holes in the PE plastic. In order to rule out the idea that the observed PE breakdown was merely the result of the masticatory system, by a mechanical activity, the researchers demonstrated that the integrity of the PE polymer surface was altered by the physical contact of the wax worm homogenate by analysing it with Fourier-transform infrared spectroscopy (FTIR) analysis, high performance liquid chromatography coupled with mass spectrometry (HPLC–MS) and Atomic Force Microscopy (AFM) [76].
Opposite results compared to Bombelli et al. [76] were found by Billen et al. [77]: carrying out the same experiment by applying a larval homogenate of G. mellonella on PE, they did not find any weight loss in PE and PS treated films, assuming that the weight loss previously reported was only due to mechanical disturbance caused during the washing of the plastic film between one application and the next [77].
However, in the following years, the capacity of G. mellonella to degrade PE has been extensively confirmed by multiple independent studies [51, 78,79,80,81,82,83].
Since these first articles, the scientific community's interest in this topic has grown more and more, and numerous papers have been published, particularly on T. molitor and G. mellonella. Almost all of the insects studied for this topic belong to the order Lepidoptera and Coleoptera (Table 1). For example, according to Peng et al. [84], Tenebrio obscurus (Coleoptera: Tenebrionidae) larvae were able to chew and consume PS; while Wang et al. [85] demonstrated the ability of Tribolium castaneum (Coleoptera: Tenebrionidae), to chew and ingest PS. Cucini et al. [86] showed how insects belonging to the species Alphitobius diaperinus (Coleoptera: Tenebrionidae) were able to ingest and apparently degrade PS; they also isolates bacteria from the gut of A. diaperinus, but poor metabolic activity was seen when cultured as a monoculture in a synthetic medium with PS [87].
Woo et al. [88] hypothesized that PS might be chemically modified and quickly degraded using Plesiophthalmus davidis (Coleoptera: Tenebrionidae) larvae and its gut microbes, since the larvae ingested PS foam and the gut microbiota formed plenty of cavities on the PS surface within 20 days of culture in a liquid-phase carbon-free medium.
After a cycle established with a diet consisting of PE or PE with waxcomb, Kundungal et al. [89] studied the subsequent generation of Achroia grisella (Lepidoptera: Pyralidae) larvae, determining that a PE only diet did not supply the nutrients needed for growth and survival. Two Indian researchers evaluated the ability of another pyralid moths, Corcyra cephalonica, to consume LDPE with or without the gut microbiome; they found out that the intact larvae ingested a small percentage more LDPE (4%), thus assuming a marginal role of the microbiome in relation to the insect itself [90]. Finally, Zhang et al.[91] isolated a bacterial strain (Klebsiella sp. EMBL-1) from the gut of another lepidopteran belonging to the Noctuidae family, Spodoptera frugiperda, fed with PVC for 5 days. According to the authors the strain was able to create a biofilm on the PVC film and used PVC polymers and monomers to produce energy for development in an efficient manner.
Since communities of microbes may be able to biodegrade plastic, researchers hypothesize that, once such a consortium has been found, it may enable the development of bioreactors for plastic breakdown [86]. Speaking of microbial consortia isolated from insect gut, Xian et al. [92] isolated two microbial consortia, one from the ocean and one from the intestine of T. molitor larvae. The two consortia were successful in using low MW additive-free PP plastics as their only carbon source for growth (low MW PP powder and amorphous PP pellets).
The bio-treated PP powder had thick biofilms and secretions on it, showing more hydroxyl and carbonyl groups, while some methyl groups were reduced, suggesting possible oxidation and deterioration [92]. Another microbial consortium was isolated from the digestive tract of T. molitor and this time it was incubated 30 days with additive-free PVC [93]. The consortium was able to use it for its growth, and upon analysis, it was discovered that the biotreated PVC had surface erosions and cracks, as well as compact biofilms on his surface, and presented high –OH and –C=C groups and low chlorine levels [93]. Thus, these works on microbial consortia present positive results, but publications on these microbial consortia and insect intestines are still few, and further studies are needed.
Focusing exclusively on the microbiome could leave out the role of the insect enzymes themselves, which could prove very useful in catalysing the plastic degradation process. Moreover, many of these researches are based on the use of genomic analysis of the 16S gene to detect changes in the microbiome of larvae subjected to different diets [94,95,96,97,98,99], and, as we will discuss in more detail in the following chapter, may mislead the research focus due to the large number of false positives [100].
Galleria mellonella
The greater wax moth G. mellonella (also known as honeycomb moth) is an invertebrate belonging to the Pyralidae family, in the Lepidoptera order. G. mellonella is a worldwide pest of bee colonies that has spread to practically all continents (excluding Antarctica) [101]. As a typical holometabolous insect, G. mellonella goes through four developmental phases throughout its life cycle: the egg, larva, pupa, and adult (Fig. 4).
It is exactly during the larval stage, when these insects are voracious eaters of bee-hive products [101], in which this lepidopteran has been seen to be able to ingest and degrade plastic polymers by various researchers [76, 78, 82, 102,103,104].
PE Degradation by Galleria mellonella
According to the paradigm set by the studies on microorganisms, plastic degradation by insects has been based on the assumption that bacteria or fungi in the animal gut were responsible for the degradation. As a consequence of this mind frame the main trend in these studies has been to investigate the role of the gut microbiome in the degradation process, with the main idea that G. mellonella larvae are able to “eat” plastic, digest it and obtain energy from it. In the scientific literature many of the efforts have focused on feeding the larvae of this lepidopteran a plastic-based diet with the aim of detecting changes in the gut microbiome of the caterpillars, which supposedly changes and adapts according to diet, or to evaluate the role of the microorganism by feeding axenic larvae a plastic diet and comparing them with untreated larvae [78, 80, 82, 103,104,105,106,107]. Below an overview of the main papers on the subject is reported.
Cassone et al. [78] treated larvae of G. mellonella with broad spectrum antibiotics in order to reduce the gut microbiota abundance and evaluate its role in the PE degradation. This approach assumed that antibiotics had no other effect whatsoever on the insect, which, to the best of our knowledge, has not been tested. Using biochemical assays, they found out that ethylene–glycol (one of the potential by-products of PE degradation [76, 108]) was considerably lower in the larval frass of the treated larvae compared with the one present in the untreated larvae, assuming decreased degradation of PE in larvae with a reduced microbiota [78]. On the other hand, using 16S amplicon sequencing, they characterized and compared the bacterial community structure of caterpillars fed with PE for 3 days to those given a natural diet (honeycomb) or no diet at all (starved) in order to better understand how ingesting and metabolizing PE alters the intestinal microbial makeup of G. mellonella. With this approach, they found out that the gut microbiota did not change and was unexpectedly consistent across time and dietary pattern. Furthermore, species diversity and richness indices showed little variation, and several taxa, that are generally found in other lepidopterans, were likewise present and well represented in G. mellonella [78], suggesting the possibility that the role of the microorganism in the PE degradation was marginal compared to the role of the own enzymes secreted by G. mellonella. In support of this thesis, in another similar study, G. mellonella was observed to degrade beeswax (which is similar to the chemical structure of PE) independently of the gut microbiome [79]: in 2019 Kong et al. [79] investigated how gut microbiome affect PE degradation. The gut microbiota was eliminated by giving second-instar larvae an antibiotic cocktail. Interestingly, the weight of the PE-fed group remained constant regardless of the impaired intestinal flora, while the weight of the group eating nutrient-dense foods steadily climbed (even if the weight increase was much smaller in antibiotic treated larvae compared to the untreated ones) and the weight of the starved group gradually declined. According to the authors, even while the gut microbiota had the capacity to contribute to the digestion of nutrient-rich foods, which contained complex nutrients, its involvement in the breakdown of PE was not as significant as that of beeswax [79]. Additionally, the fact that the same long-chain fatty acids and related metabolites were found in the larval frass regardless of the intestinal microbiota presence or absence, supported the idea that this did not manufacture long-chain fatty acids or long-chain ethanol by breaking down long-chain hydrocarbon wax. These data suggested the idea that the gut microbiota did not play a fundamental role in the degradation of wax (and long hydrocarbon-chain in general) into long-chain fatty acids [79].
Ren et al. [80] isolated the bacteria Enterobacter sp. D1 from the intestinal homogenate of the wax moth by employing PE as the only carbon source. Additional research verified that strain D1 could break down PE; however, at the laboratory level, neither microorganisms nor degrading enzymes were able to produce the desired breakdown effect of PE [80]; these results suggested, again, that G. mellonella metabolism plays an important role in the degradation of plastic.
Following PE and beeswax co-feeding or beeswax feeding, Latour et al. [103] looked into the intestinal bacterial population of G. mellonella larvae. The predominant bacterial families identified by high throughput 16S rRNA sequencing were Enterococcaceae and Oxalobacteraceae, with Enterococcus as the most prevalent represented genus; indeed, Enterococcus has the highest abundance in the microbiome for many lepidopterans like Spodoptera littoralis, Hyles euphorbiae, Brithys crini, Bombyx mori or Plutella xylostella [109,110,111,112]. The Corynebacterium and Curvibacter genera were among the most numerous in both samples, but no difference was found in the bacterial community between the two groups [103]. In addition, the authors stated that several bacteria-associated enzymes, such phenylacetaldehyde dehydrogenase, may play potential roles in the breakdown of plastics [103]. Noel et al. [113] also analyzed the gut flora of wax-fed larvae compared with that of larvae fed with wax + LDPE (via Amplicon technology); no significant bacterial community differences were found between the two diets and the two largest bacterial families were discovered to be once again Enterococcaceae and Oxalobacteraceae, and once more Corynebacterium was found in LDPE fed larvae [113]. Corynebacteriaceae have been highlighted for their ability to breakdown PE [114]. Réjasse et al. [105] investigated the role of G. mellonella in biodegrading PE and also assessed if the larvae were able to bioassimilate PE. In this study early stages of the larva (L2–L3) fed with PE lost weight and perished in 3 (50%) to 10 days (100%) [104]. This pattern reflected the findings of another study (50% death at 15 days) [82], while other researchers reported a 20 to 50% weight loss in larvae bred with a PE diet [79, 104]. In comparison to larvae fed with pollen or beeswax, L6 larvae fed exclusively with PE lost weight and ate 80 times less food [105]. Réjasse et al. [105] found no discernible variations in weight gain or feeding behavior between conventional and axenic larvae, indicating that the microbiota might not be crucial to their growth and development, the same result obtained by Kong et al. [79, 105]. Researchers also evaluated if PE could be bioassimilated by G. mellonella larvae by μFTIR hyperspectral imaging of carbon − deuterium bonds in cryosections of caterpillars fed with PE isotopically labeled with deuterium (PED4); the findings indicated that neither axenic nor conventional larvae had undergone PE bio assimilation [105]. However, they discovered weak oxidation in PE films exposed for 24 h to the dissected stomach of typical larvae as well as a shortening of aliphatic chains in PED4 particles excreted in the larval frass, indicating that G. mellonella was capable of PE oxidation [105]. Since oxidation is the initial stage of environmental biodegradation, the oxidation of PE in the larval gut may promote its degradation even in the absence of bioassimilation [105].
According to another study on the effect of LDPE diet on G. mellonella, a meal consisting solely of LDPE could partially support the sustenance and metabolic processes of the larval fat body [104]. Although plastic-fed caterpillars could retain their lipid reserves, they were obviously lacking in many other metabolites (such as amino acids and carbohydrates), which probably contributed to their lower survival and development rates [104].
The obtained data suggests that in order for the utilization of this system for plastic bioremediation to be sustainable, additional nutritional intake should be needed [104]; this lack of nutrients in plastic diets was confirmed by the survival rate and grow rate of larvae fed with plastics found out by other authors [102, 105, 107, 115]; indeed this whole field points towards an impaired growth of the larvae on diet composed only of plastic, with their inability to derive energy and thrive on a PE diet. By biochemical approaches researchers discovered that potential lipid oxidation enzymes, such as alcohol dehydrogenase, had significantly increased activity in PE-fed larvae, but this could be as a result of an increase in the fatty acid metabolism to make up for the absence of other nutrients in plastic [104].
Difference in larval microbiota when fed with plastic (PE and PS) compared to the ones fed with beeswax was found by Ruiz Barrionuevo et al. [106]. Researchers found out that bacteria and fungus in the gut microbiome of wax moths responded differently to feed. Microbiome from beeswax and plastic-based diets had different bacterial compositions; in the beeswax diet, Streptococcus, Porphyromonas, or Fusobacteria predominated. In contrast to bacteria, the composition of fungal communities did not change according to food, although there were considerable variations in richness and variety. This study showed that bacterial communities were more susceptible to dietary changes than fungal communities, which simply experienced changes in fungal relative abundance. In this study, it was shown that the guts of larvae fed with plastic had high concentrations of several Pseudomonas (Pseudomonadales), Bradyrhizobium (Rhizobiales), and Comamonadaceae (Burkholderiales) populations [106]. A similar study was published by Peydaei et al. [115], in which they reared larvae of G. mellonella on a PE, PS and PP diet for 8 days and then they characterized the microbial communities of the salivary glands, the intestine and the whole insect by high throughput sequencing of 16S rRNA analysis. They observed that, while it did not seem to have an impact on the microbial population in the salivary glands, the mastication of expanded PS (EPS), PP, or PE might change the gut microbiome. Desulfovibrio vulgaris and Enterobacter species proliferated with the PE diet, while Enterococcus rose when PS and PP were chewed, while EPS diet enhanced the number of Paenibacillus, Corynebacterium, and Commamonadaceae [115].
Kundungal et al. [81] studied the effect that a UV pre-treatment of LDPE had on its consumption by G. mellonella. When comparing UV-treated plastic to non-treated plastic, PE consumption increased by up to 37%; moreover, larvae given the control diet and pre-treated LDPE had a 100 ± 0% survival rate. However, waxworms fed untreated LDPE had a much lower survival rate (73.40 ± 2.50%). Finally, due to the combined effects of photodegradation and degradation via waxworm digestion, pre-treated LDPE was degraded more efficiently than untreated LDPE [81].
Despite all the studies focusing on the gut microbiome of G. mellonella the results obtained were very weak and inconsistent: the identified microorganisms did not show PE degrading ability in isolation with plastic, and any sure enzyme involved in plastic degradation has not been identified.
The approach based on the idea that the worms eat plastics, and therefore the gut microorganism play a paramount role, is adopted by many researchers, as we can see from Table 2. This concept presents two faults. The first aspect regards the idea that animals might survive and change their microbiota in response to an only plastic diet. The second problematic aspect is related to the bioinformatic tools used to identify microorganism sequences. In fact, the accuracy of some of these analyses should be questioned, as recently highlighted by Serrano et al. [100]. In this study the researchers developed a computational framework to assess the effectiveness of metagenomic investigations based on the creation of synthetic microbiomes that mimicked actual bacterial communities. Using this method, they were able to pinpoint significant gaps in the ability of the technologies that are now available to define microbiomes. According to the authors, the inability of 16S and whole genome sequencing (WGS) analysis to identify species that are not included in genomic databases is obvious but, unexpectedly, the lack of complete information also increases the detection of false positives, which may seriously affect the identification of the species present in a microbiome [100]. These restrictions severely impede the ability to successfully identify the bacteria present in various communities, with identification instead of false positives, including families and genus of bacteria originally absent from the sample. The misinterpretation of the data can generate false leads which are more often than not used as guidelines and references in the field.
The difficulty to accurately characterize microbiomes critically constrains the ability of currently available techniques to detect the potential changes in the composition of microbiomes induced by changes in the insects' diet. Most of the detected changes can be spurious, which questions the utility of these techniques to identify the microorganisms that respond to a given treatment of the host, in particular those supposed to thrive on a plastic diet [100].
Overall, this trend and the obtained results raise questions about the commonly adopted experimental method [78, 82, 103, 104, 113, 115], based on changes in bacterial abundance (for example providing diverse diets) to find microbes with certain metabolic potentials [100]. On the other hand, in studies attempting to isolate PE degrading bacteria from the gut of larvae, as in Ren et al. [80], the results of this degradation were not satisfactory when compared to the activity carried out by the insect in vivo.
Within this scenario it is imperative to mention that the anatomy and physiology of the animal is too often neglected. Plastic based diets are usually compared with starvation and if weight loss is greater in the case of starvation, then it is assumed that the worms are able to feed only on plastic. However, other aspects of the worm metabolic activity are not considered: for example, if a worm weight in presence of plastic does not decrease as much as when in starvation might mean that in the absence of any potential food to chew on, an unknown metabolic cascade (autophagy for example) could be activated. Hence the difference in weights.
Finally, in many of the studies mentioned, the feeding of plastic-based diet occurs for short periods, generally 5 to 10 days [102, 104, 105, 115, 121, 122], and this methodology may not be reliable in assessing the survival and growth rate of the larvae subjected to this peculiar diet; in this case, it may be more accurate to conduct trials with longer periods of plastic-based diet feeding, assessing larval growth, survival, ability to pupate and ability to reproduce of the adults, possibly investigating, using the same methodology, the following generations; indeed, it would be rash to claim that larvae are able to feed and take energy from plastic when precisely these tests are conducted for short periods of time.
In this unexplored landscape it is definitely worth considering that the larval alimentary canal of lepidopterans, is essentially a straightforward tube that extends the full length of the animal [125] and lacks both specialized chambers for digestion, and a specific microbiome [126].
Despite the results of the studies mentioned above, there are still few studies that focus exclusively on the role of the insect, leaving out that of the microbiome and do not focus on the survival rate and the ability of the larvae to ingest plastic. In 2020 a group of researchers carried out a proteomic investigation on the glands collected from larvae fed with PE for 10 days. They found out that, when exposed to PE, there was an increase in protein synthesis linked to fatty acid beta-oxidation, and there was a downregulation of juvenile hormone esterase (JHE), that could suggest a prolongation of the last larval instar stage most likely due to a general decline in metabolism, which implies that the larvae encounter a lower energy level during ingestion and digestion of PE. FTIR analysis carried out on UV pre-treated PE sample incubated with salivary glands or gut suspensions for 20 days at 32 °C revealed the presence of peaks related to fatty acids composition, along with a strong and broad peak at 3300 cm−1 that corresponds to the peak caused by OH stretching in ethylene glycol [116].
However, all these data did not answer the key question, regarding the mechanism of plastic degradation by the wax worm.
A turning point in research came in 2022 with the study by Sanluis-Verdes et al. [83] who were able to identify for the first-time active molecules capable of oxidizing PE without any prior treatment.
G. mellonella PEases
Published in 2022 by Sanluis-Verdes et al. [83], this was the first work in which enzymes from the animal kingdom were shown to be able to oxidize PE, paving the way for bio-recycling and up-cycling as potential solutions for managing plastic waste. Here the wax worm saliva (defined as the juice present in the first part of the digestive system) was collected and tested on commercial PE film. The results demonstrated that PE exposure to G. mellonella saliva caused PE to oxidize and depolymerize within a few hours of exposure at room temperature, resulting in the production of oxidized molecules with low MW, while no oxidation was produced when the control saliva of a different lepidopteran larva, Samia cynthia, was added to the PE film [83]. The saliva was able to overcome a recognized bottleneck step (i.e., oxidation) [127, 128] in PE degradation. Therefore, the effect of G. mellonella saliva on PE was equivalent to that of abiotic pre-treatments and the saliva-dependent oxidation of PE might serve as an appropriate substrate for subsequent biological treatment [83]. A proteomic analysis was also carried out on the wax worm saliva in order to identify the enzymes therein contained responsible for PE oxidation. Two enzymes were then reported to be responsible for the degradation of PE, an arylphorin and an hexamerin, renamed by the researchers PEases, since their ability to oxidize PE. These two proteins are phylogenetically connected to phenol oxidases (enzymes which target aromatic rings) and hemocyanins, an oxygen transport protein that also has phenoloxidase activity [129, 130]. Among the detected breakdown products, a tiny aromatic molecule that may be recognized as a plastic additive was detected, suggesting that this substance may end up being the target of the larval enzyme phenoloxidase-like activity and it was hypothesized that the production of free radicals and subsequent start of the autoxidative chain reaction could be a result of the wax worm enzyme effect on aromatic additives [83]. Free radical production as a precursor to auto oxidation is not a novel concept [131, 132]. The ability of enzymes to degrade PE and leave the polymer more susceptible to enzymatic degradation without any abiotic pre-treatment was reported in this study for the first time [83]. Furthermore, this research raised the possibility that insect saliva could act as a reservoir for enzymes that break down materials (such as plastic, cellulose, or lignin), which might completely alter the bioremediation industry. This result suggests a different possible strategy for dealing with plastic degradation, even if more research will be required to fully understand the sequential progression of plastic in interaction with various saliva enzymes [83]. What molecular process is responsible for this enzymatic oxidation is still unsure. Bertocchini & Arias [133] try to answer this question by investigating the natural role of these phenol oxidases enzymes. The larval ability to neutralize phenolic chemicals enables them to feed on leaves or other plant products (pollen, resins, etc.) that contain these molecules that are usually used by plants as a defence mechanism against plants eating organisms [134]. Certain plastic additives resemble plant phenolic chemicals and, in theory, may end up being targets of G. mellonella phenol oxidases, which may produce free radicals and eventually cause the polymer to oxidize. However, the authors did not rule out that this deterioration was caused by the more classic enzymatic cleavage of the C–C bound in the aliphatic chain [133].
This study confirmed the crucial role that G. mellonella’s enzymes play in the breakdown of PE (since the saliva of the worms and the enzymes contained in it, oxidize and depolymerize PE) [82], as, due to the fast breakdown rate, it seemed doubtful that the larvae’s gut bacteria was the only factor contributing to the observed alteration in the chemical structure of PE [79].
Degradation of Plastics Other than PE by G. mellonella
Currently, studies on the degradation of plastics by G. mellonella focus mainly on PE, but other types of plastics are also evaluated.
For example, Venegas et al. [123] investigated the potential degradation PS carried out by G. mellonella. By feeding this polymer to the larvae, researchers attempted to identify potential enzyme candidates for the breakdown of PS. By using FTIR-Attenuated Total Reflectance (ATR) they confirmed PS degradation and through liquid chromatography with tandem mass spectrometry (LC–MS/MS) they were able to detect 27 potential responsible enzymes; of these, two showed similarities with possible styrene-degrading enzymes already known. The authors suggested that a PS diet could induce changes in the protein background of the gut of G. mellonella larvae, with the presence of an important group of oxidoreductase enzymes [123].
Wang et al. [118] demonstrated that PS could be efficiently biodegraded by the G. mellonella larvae. The in vivo depolymerization of PS was confirmed by SEM analysis of the larva intestine and by the presence of styrene (the monomer of PS) in the metabolites of the treated larvae. Moreover, step-by-step chain reactions were used to suggest two potential PS metabolic pathways in the intestines of G. mellonella larvae: the styrene oxide–phenylacetaldehyde and 4-methylphenol-4-hydroxybenzaldehyde-4-hydroxybenzoate pathways. The styrene oxide–phenylacetaldehyde, together with the styrene cis-glycol-3-vinylcatechol pathway, were already being suggested by other authors as possible pathways for the degradation of PS, but without empirical data to support it [2, 135]. In this paper, by looking for the corresponding metabolites, the styrene oxide-phenylacetaldehyde pathway was confirmed [118]. Finally, Wang et al. showed that PS polymers can be successfully depolymerized in G. mellonella larvae treated with antibiotics, proving once again that the in vivo depolymerization of PS was not strictly dependent on the presence of gut microbes. By analyzing the types and contents of the metabolites in antibiotic-treated larvae, they suggested that a healthy gut microbiota could be beneficial for the oligomer or monomer metabolism of PS [118].
In 2021 Zhu et al. [117] published a study regarding the degradation of plastics from waste electrical and electronic equipment (WEEE). They fed G. mellonella larvae with waste rigid polyurethane (WRPU), waste polystyrene (WPS) and waste acrylonitrile–butadiene–styrene (WABS). Once again, they demonstrated that G. mellonella larvae are able to chew and degrade to some extent this kind of plastic waste. The number average MW (Mn) (which is the statistical mean MW of all polymer chains in the given sample) and weight average MW (Mw) (that takes into account the MW of a chain when evaluating contributions to the MW average) in the WPS group and the WABS group, decrease by 12.23% and 8.11% and 28.37% and 0.52%, respectively, indicating that the MW of the WEEE plastics moved toward the low MW. Additionally, the fate of WRPU in the bodies of G. mellonella larvae was investigated using fluorescence imaging and the result was that, within 24 h, the total fluorescence intensity of WRPU in the gut of G. mellonella larvae decreased from 5.904 to 5.140*109. Finally based on 16S rRNA gene sequencing analysis of the intestinal microbial communities, the authors stated that Enterobacter was primarily responsible for the breakdown of WRPU, whereas Enterococcus was more responsible for the breakdown of WABS and WPS [117]. Jiang et al. also evaluated the changes in the intestinal microbiota caused by the PS diet: superworm and yellow mealworm community diversity decreased, while G. mellonella community diversity increased. Researchers hypothesized that PS degradation may be significantly aided by Enterococcus and Enterobacteriaceae, since they were found in the larvae of all three species after 20 days of PS feeding [95]. However, these results, taking into account the findings exposed by Serrano et al. [100] in which meta genomic analyses were used to detect changes in the relative abundance of bacterial groups based on the diet, had a high probability to be misleading: the data should be interpreted with care and, eventually, confirmed by testing the effect of a single culture of the bacterial strain of election on plastic.
A work regarding the consuming preference of G. mellonella larvae over different types of plastic was published in 2022 by Zhu et al. [122]. Two different types of WEEE plastics (WRPU and WPS) and some pristine plastics (PE, PP, PVC, RPU and PS) were used; larvae exhibited lower consumption preferences for WRPU and WPS over the corresponding virgin plastics, possibly as a result of the higher chlorine or metal contents in the plastics due to the absorption during their usage. G. mellonella ingestion of PE was higher than that of PS, in contrast T. molitor larvae revealed the opposite pattern [122]. A higher PS consumption by yellow mealworms over honeycomb moth was found in another work, in which T. molitor caused the mass of EPS to drop by 54%, while in PVC the decrease was 10% and in PET 12%, a better result compared to the 18% and 2% mass loss reached by G. mellonella for EPS and PET respectively; however, G. mellonella showed a higher consumption of PVC (34%) [119], but it is important to mention that in this work only the mass loss of the various type of plastic was evaluated and it is possible that this was only ingested without actual degradation of it.
Burd et al. [121] observed that, after 7.25 days of exposure to PS and LDPE diets, the larvae had consumed 56.12% of PS, but only 5.11% of LDPE; the degradation was then confirmed by FTIR analysis for the PS, while, unlike the other papers, for PE the results of the analysis indicated low or almost no degradation [121].
A recent study showed that the G. mellonella larvae exhibited differential plastic consumption, with a preference for single- and triple-layered PS and PE over biaxially oriented PP. According to the authors, diets made of plastic had an impact on survival rates and changes in developmental stages [107]: larvae fed with plastics pupated sooner than larvae fed with beeswax, as also reported by Burd et al. [121], which is probably due to a stress response to a very poor diet [136], reinforcing the idea that a plastic-based diet is nutritionally unsuitable for the caterpillar growth.
Recently the effect of a PP diet on G. mellonella was investigated. Over the course of 12 days, 50 larvae ingested roughly 133.4 mg of PP film with an average consumption rate of 1.75 mg per 1 g of larvae for the 12 days [124]. Then, after two years of growing the gut microbiota, they were able to isolate Bacillus cereus; the authors proposed that this microorganism possesses the full complement of enzymes necessary to start PP carbon chain oxidation, a crucial step in degradation. They used Scanning Electron Microscopy—Energy Dispersive X-ray spectroscopy (SEM–EDS), FTIR, and X-ray photoelectron spectroscopy (XPS) to show that both the gut bacteria and B. cereus could individually start the oxidation of PP after a 30-day incubation at 37 °C. According to the authors high temperature gel permeation chromatography (HT-GPC) analysis demonstrated that B. cereus biodegraded PP by wide depolymerization; however, the small molecules resulting from the biodegradation, were absent in the reported GPC results [124].
Using transmission electron microscopy (TEM), confocal microscopy and flow cytometry, Rost-Roszkowska et al. [120] showed that on G. mellonella larvae, fed with PP bags for 24 and 48 h, the ultrastructure of internal organs was unaffected by such diet. The cells in the examined organs (the midgut, the silk gland, and the fat body) exhibited no signs of degenerative changes [120]. Usually, animals may suffer several fatal consequences from consuming plastics, which could harm the cells in their tissues and organs [137,138,139,140,141], but this did not occur in PP fed larvae of G. mellonella. However, it is important to underline how these tests were conducted with larvae subjected to this peculiar diet for 24 and 48 h, which could be too short a time to evaluate this type of changes.
Conclusions
Because of its portability, low cost, and ease of processing, plastic items are utilized extensively around the world. Unfortunately, the increase in plastic production together with an improper plastic product disposal have so far resulted in significant pollution, with no reliable plan set up to deal with the urgent plastic waste pollution issue. The degradation of plastics by G. mellonella shows great promise as a potential solution to the global plastic pollution crisis. The amount of insect species reported to chew and degrade plastic has been growing, but currently, T. molitor and G. mellonella are the most researched insects (as evidenced also by Pivato et al. [48]) and, apparently, the most efficient even if, as evidenced by some authors, different insects can be more efficient in helping to degrade different plastics [122]. This lepidopteran has undoubtedly demonstrated his ability to efficiently degrade various types of plastics, including PE and PS, making it a viable candidate for the development of new strategies in the plastic waste disposal. Most studies have focused and still focus on the sole role of microorganisms in biodegrading plastic, considering it the number one responsible for this process, but the results are controversial. Recently various studies have shown that the role of microbiota in the degradation process of synthetic polymers carried out by G. mellonella is secondary compared to the one played by the insect itself. A recent study by Sanluis-Verdes et al. [83] has identified two active molecules responsible for PE oxidation and depolymerization: by introducing an oxygen molecule and thus oxidizing the polymer, these proteins can help overcome the bottleneck of plastic degradation. This opens the way to new investigations that need to focus more on the role of the of the insects’ enzymes, since, regardless of many studies that have recently been published regarding microbe species that may be accountable for insect-driven plastic degradation, little to no conclusion has been achieved on the precise species or genera of bacteria/fungi that populate the Lepidoptera and Coleoptera intestinal tract and are supposedly engaged in plastic degradation [51, 78, 80, 82, 95, 104, 106, 117].
Overall, the findings from the studies here analyzed suggest that G. mellonella has the potential to be a valuable tool in the fight against plastic pollution but results are nowhere near their practical implementation and further research is needed to make the most of what insects can offer to improve the problem of plastic pollution.
Abbreviations
- PE:
-
Polyethylene
- LDPE:
-
Low-density polyethylene
- LLDPE:
-
Linear low-density polyethylene
- PED4:
-
Polyethylene isotopically labeled with deuterium
- PP:
-
Polypropylene
- PVC:
-
Polyvinyl chloride
- PET:
-
Polyethylene terephthalate
- PLA:
-
Polylactic acid
- PMMA:
-
Poly (methyl methacrylate)
- PU:
-
Polyurethane
- RPU:
-
Rigid polyurethane
- PS:
-
Polystyrene
- EPS:
-
Expanded polystyrene
- CARG:
-
Compound Annual Growth Rate
- MW:
-
Molecular weight
- VOCs:
-
Volatile organic compounds
- FTIR:
-
Fourier-transform infrared spectroscopy
- H NMR:
-
Proton nuclear magnetic resonance
- HPLC–MS:
-
High performance liquid chromatography-mass spectrometry
- AFM:
-
Atomic Force Microscopy
- NGS:
-
Next-generation sequencing
- GC–MS:
-
Gas chromatography–mass spectrometry
- JHE:
-
Juvenile Hormone Esterase
- LC–MS/MS:
-
Liquid Chromatography with tandem mass spectrometry
- ATR:
-
Attenuated Total Reflectance
- WGS:
-
Whole genome sequencing
- WEEE:
-
Waste electrical and electronic equipment
- WRPU:
-
Waste rigid polyurethane
- WPS:
-
Waste polystyrene
- WABS:
-
Waste acrylonitrile–butadiene–styrene
- TG-MS:
-
Thermal Gravimetric-Mass Spectrometry
- PF:
-
Phenol–formaldehyde resin
- SEM–EDS:
-
Scanning Electron Microscopy-Energy Dispersive X-ray spectroscopy
- XPS:
-
X-ray photoelectron spectroscopy
- TEM:
-
Transmission electron microscopy
- Mn:
-
Number average MW
- Mw:
-
Weight average MW
Reference
Ru J, Huo Y, Yang Y (2020) Microbial degradation and valorization of plastic wastes. Front Microbiol 11:442. https://doi.org/10.3389/fmicb.2020.00442
Ali SS, Elsamahy T, Koutra E et al (2021) Degradation of conventional plastic wastes in the environment: a review on current status of knowledge and future perspectives of disposal. Sci Total Environ 771:144719. https://doi.org/10.1016/j.scitotenv.2020.144719
(2023) Market size value of plastics worldwide from 2021 to 2030. In: Statista Research Department. https://www.statista.com/statistics/1060583/global-market-value-of-plastic/. Accessed 25 May 2023
Plastics Europe (2022) Plastic—The Facts 2022
(2023) Annual production of plastics worldwide from 1950 to 2021. In: Statista Research Department. https://www.statista.com/statistics/282732/global-production-of-plastics-since-1950/. Accessed 25 May 2023
Chamas A, Moon H, Zheng J et al (2020) Degradation rates of plastics in the environment. ACS Sustain Chem Eng 8:3494–3511. https://doi.org/10.1021/acssuschemeng.9b06635
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Phil Trans R Soc B: Biol Sci 364:1985–1998. https://doi.org/10.1098/rstb.2008.0205
Jambeck JR, Geyer R, Wilcox C et al (2015) Plastic waste inputs from land into the ocean. Science 347:768–771. https://doi.org/10.1126/science.1260352
Hurley R, Horton A, Lusher A, Nizzetto L (2020) Plastic waste in the terrestrial environment. In: Plastic Waste and Recycling. Elsevier, pp 163–193
Blettler MCM, Wantzen KM (2019) Threats underestimated in freshwater plastic pollution: mini-review. Water Air Soil Pollut 230:174. https://doi.org/10.1007/s11270-019-4220-z
Zubris KAV, Richards BK (2005) Synthetic fibers as an indicator of land application of sludge. Environ Pollut 138:201–211. https://doi.org/10.1016/j.envpol.2005.04.013
Rillig MC (2012) Microplastic in terrestrial ecosystems and the soil? Environ Sci Technol 46:6453–6454. https://doi.org/10.1021/es302011r
Wagner M, Scherer C, Alvarez-Muñoz D et al (2014) Microplastics in freshwater ecosystems: what we know and what we need to know. Environ Sci Eur 26:12. https://doi.org/10.1186/s12302-014-0012-7
Zalasiewicz J, Waters CN, Ivar do Sul JA, et al (2016) The geological cycle of plastics and their use as a stratigraphic indicator of the Anthropocene. Anthropocene 13:4–17. https://doi.org/10.1016/j.ancene.2016.01.002
Davis H (2015) Life & Death in the Anthropocene: A Short History of Plastic. In: Art in the anthropocene: Encounters among aesthetics, politics, environments and epistemologies. pp 347–358
European Bioplastics (2016) FACT SHEET: What are bioplastics?
Reddy RL, Reddy VS, Gupta GA (2013) Study of bio-plastics as green and sustainable alternative to plastics. Int J Emerg Technol 3:82–89
Bioplastics market data. In: Europeanbioplastics. https://www.european-bioplastics.org/market. Accessed 25 May 2023
Rahman MH, Bhoi PR (2021) An overview of non-biodegradable bioplastics. J Clean Prod 294:126218. https://doi.org/10.1016/j.jclepro.2021.126218
Vollmer I, Jenks MJF, Roelands MCP et al (2020) Beyond mechanical recycling: giving new life to plastic waste. Angew Chem Int Ed 59:15402–15423. https://doi.org/10.1002/anie.201915651
Ali SS, Elsamahy T, Al-Tohamy R et al (2021) Plastic wastes biodegradation: mechanisms, challenges and future prospects. Sci Total Environ 780:146590. https://doi.org/10.1016/j.scitotenv.2021.146590
Okan M, Aydin HM, Barsbay M (2019) Current approaches to waste polymer utilization and minimization: a review. J Chem Technol Biotechnol 94:8–21. https://doi.org/10.1002/jctb.5778
MacArthur E (2017) Beyond plastic waste. Science 358:843–843. https://doi.org/10.1126/science.aao6749
Rahimi A, García JM (2017) Chemical recycling of waste plastics for new materials production. Nat Rev Chem 1:0046. https://doi.org/10.1038/s41570-017-0046
Zhang F, Zhao Y, Wang D et al (2021) Current technologies for plastic waste treatment: a review. J Clean Prod 282:124523. https://doi.org/10.1016/j.jclepro.2020.124523
(2023) Plastics: Material-Specific Data. In: United States Environmental Protection Agency. https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data. Accessed 25 May 2023
Raddadi N, Fava F (2019) Biodegradation of oil-based plastics in the environment: existing knowledge and needs of research and innovation. Sci Total Environ 679:148–158. https://doi.org/10.1016/j.scitotenv.2019.04.419
Wei X-F, Bohlén M, Lindblad C et al (2021) Microplastics generated from a biodegradable plastic in freshwater and seawater. Water Res 198:117123. https://doi.org/10.1016/j.watres.2021.117123
(2022) Global plastic waste set to almost triple by 2060. In: Organisation for Economic Cooperation and Development (OECD). https://www.oecd.org/environment/global-plastic-waste-set-to-almost-triple-by-2060.htm. Accessed 25 May 2023
Zhang K, Hamidian AH, Tubić A et al (2021) Understanding plastic degradation and microplastic formation in the environment: a review. Environ Pollut 274:116554. https://doi.org/10.1016/j.envpol.2021.116554
Liu K, Wang Z, Zhang Y et al (2019) Vapour-liquid equilibrium measurements and extractive distillation process design for separation of azeotropic mixture (dimethyl carbonate + ethanol). J Chem Thermodyn 133:10–18. https://doi.org/10.1016/j.jct.2019.01.027
Bracco P, Costa L, Luda MP, Billingham N (2018) A review of experimental studies of the role of free-radicals in polyethylene oxidation. Polym Degrad Stab 155:67–83. https://doi.org/10.1016/j.polymdegradstab.2018.07.011
Pirsaheb M, Hossini H, Makhdoumi P (2020) Review of microplastic occurrence and toxicological effects in marine environment: experimental evidence of inflammation. Process Saf Environ Prot 142:1–14. https://doi.org/10.1016/j.psep.2020.05.050
Kamweru PK, Ndiritu FG, Kinyanjui TK et al (2011) Study of temperature and UV wavelength range effects on degradation of photo-irradiated polyethylene films using DMA. J Macromol Sci, Part B 50:1338–1349. https://doi.org/10.1080/00222348.2010.516172
Pal P, Pandey JP, Sen G (2018) Synthesis and Application as Programmable Water Soluble Adhesive of Polyacrylamide Grafted Gum Tragacanth (GT-g-PAM). In: Biopolymer Grafting: Applications. Elsevier, pp 153–203
Crawford CB, Quinn B (2017) Physiochemical properties and degradation. In: Microplastic Pollutants. Elsevier, pp 57–100
Polyolefins. In: Plastics Europe. https://plasticseurope.org/plastics-explained/a-large-family/polyolefins-2/. Accessed 25 May 2023
Elgharbawy AS, Ali RM (2022) A comprehensive review of the polyolefin composites and their properties. Heliyon 8:e09932. https://doi.org/10.1016/j.heliyon.2022.e09932
Chen X, Xiong X, Jiang X et al (2019) Sinking of floating plastic debris caused by biofilm development in a freshwater lake. Chemosphere 222:856–864. https://doi.org/10.1016/j.chemosphere.2019.02.015
Singh B, Sharma N (2008) Mechanistic implications of plastic degradation. Polym Degrad Stab 93:561–584. https://doi.org/10.1016/j.polymdegradstab.2007.11.008
Ehrenstein GW (2001) Polymeric materials: structure, properties, applications. Hanser Publishers, München
Queste BY, Fernand L, Jickells TD, Heywood KJ (2013) Spatial extent and historical context of North Sea oxygen depletion in August 2010. Biogeochemistry 113:53–68. https://doi.org/10.1007/s10533-012-9729-9
Pischedda A, Tosin M, Degli-Innocenti F (2019) Biodegradation of plastics in soil: the effect of temperature. Polym Degrad Stab 170:109017. https://doi.org/10.1016/j.polymdegradstab.2019.109017
Booth AM, Kubowicz S, Beegle-Krause C, et al (2017) Microplastic in global and Norwegian marine environments: distributions, degradation mechanisms and transport. Trondheim: Agency, N.E., M-918|2017
Pitt CG (1992) Non-microbial degradation of polyesters: mechanisms and modifications. Spec Publ—R Soc Chem 109:7–7
(2014) Biodegradation. In: The IUPAC Compendium of Chemical Terminology. International Union of Pure and Applied Chemistry (IUPAC), Research Triangle Park, NC
Lu H, Diaz DJ, Czarnecki NJ et al (2022) Machine learning-aided engineering of hydrolases for PET depolymerization. Nature 604:662–667. https://doi.org/10.1038/s41586-022-04599-z
Pivato AF, Miranda GM, Prichula J et al (2022) Hydrocarbon-based plastics: progress and perspectives on consumption and biodegradation by insect larvae. Chemosphere 293:133600. https://doi.org/10.1016/j.chemosphere.2022.133600
Sharma H, Neelam DK (2023) Understanding challenges associated with plastic and bacterial approach toward plastic degradation. J Basic Microbiol 63:292–307. https://doi.org/10.1002/jobm.202200428
Kjeldsen A, Price M, Lilley C, et al (2019) Review of Standards for Biodegradable Plastics. Industrial Biotechnology Innovation Centre
Montazer Z, Habibi Najafi MB, Levin DB (2021) In vitro degradation of low-density polyethylene by new bacteria from larvae of the greater wax moth, Galleria mellonella. Can J Microbiol 67:249–258. https://doi.org/10.1139/cjm-2020-0208
Montazer Z, Habibi Najafi MB, Levin DB (2020) Challenges with verifying microbial degradation of polyethylene. Polymers (Basel) 12:123. https://doi.org/10.3390/polym12010123
Morohoshi T, Oi T, Aiso H et al (2018) Biofilm Formation and degradation of commercially available biodegradable plastic films by bacterial consortiums in freshwater environments. Microbes Environ 33:332–335. https://doi.org/10.1264/jsme2.ME18033
Urbanek AK, Rymowicz W, Mirończuk AM (2018) Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl Microbiol Biotechnol 102:7669–7678. https://doi.org/10.1007/s00253-018-9195-y
Pathak VM, Navneet, (2017) Review on the current status of polymer degradation: a microbial approach. Bioresour Bioprocess 4:15. https://doi.org/10.1186/s40643-017-0145-9
Sivan A, Szanto M, Pavlov V (2006) Biofilm development of the polyethylene-degrading bacterium Rhodococcus ruber. Appl Microbiol Biotechnol 72:346–352. https://doi.org/10.1007/s00253-005-0259-4
Fontanella S, Bonhomme S, Koutny M et al (2010) Comparison of the biodegradability of various polyethylene films containing pro-oxidant additives. Polym Degrad Stab 95:1011–1021. https://doi.org/10.1016/j.polymdegradstab.2010.03.009
Santo M, Weitsman R, Sivan A (2013) The role of the copper-binding enzyme—laccase—in the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int Biodeterior Biodegradation 84:204–210. https://doi.org/10.1016/j.ibiod.2012.03.001
Ward PG, Goff M, Donner M et al (2006) A two step chemo-biotechnological conversion of polystyrene to a biodegradable thermoplastic. Environ Sci Technol 40:2433–2437. https://doi.org/10.1021/es0517668
Gilan I, Hadar Y, Sivan A (2004) Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl Microbiol Biotechnol. https://doi.org/10.1007/s00253-004-1584-8
Hu B, Wang M, Geng S et al (2020) Metabolic exchange with non-alkane-consuming Pseudomonas stutzeri SLG510A3–8 improves n -alkane biodegradation by the alkane degrader Dietzia sp. Strain DQ12–45–1b. Appl Environ Microbiol. https://doi.org/10.1128/AEM.02931-19
Roberts C, Edwards S, Vague M et al (2020) Environmental consortium containing Pseudomonas and Bacillus Species synergistically degrades polyethylene terephthalate plastic. mSphere. https://doi.org/10.1128/mSphere.01151-20
Edwards S, León-Zayas R, Ditter R et al (2022) Microbial consortia and mixed plastic waste: pangenomic analysis reveals potential for degradation of multiple plastic types via previously identified PET degrading bacteria. Int J Mol Sci 23:5612. https://doi.org/10.3390/ijms23105612
Esmaeili A, Pourbabaee AA, Alikhani HA et al (2013) Biodegradation of Low-density polyethylene (LDPE) by mixed culture of Lysinibacillus xylanilyticus and Aspergillus niger in soil. PloS one 8:e71720. https://doi.org/10.1371/journal.pone.0071720
Skariyachan S, Patil AA, Shankar A et al (2018) Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym Degrad Stab 149:52–68. https://doi.org/10.1016/j.polymdegradstab.2018.01.018
Muenmee S, Chiemchaisri W, Chiemchaisri C (2016) Enhancement of biodegradation of plastic wastes via methane oxidation in semi-aerobic landfill. Int Biodeterior Biodegradation 113:244–255. https://doi.org/10.1016/j.ibiod.2016.03.016
Muhonja CN, Makonde H, Magoma G, Imbuga M (2018) Biodegradability of polyethylene by bacteria and fungi from Dandora dumpsite Nairobi-Kenya. PloS one 13:e0198446. https://doi.org/10.1371/journal.pone.0198446
Zhang N, Ding M, Yuan Y (2022) Current advances in biodegradation of polyolefins. Microorganisms 10:1537. https://doi.org/10.3390/microorganisms10081537
Arkatkar A, Arutchelvi J, Sudhakar M et al (2009) Approaches to Enhance the Biodegradation of Polyolefins. Open Environ Eng J 2:68–80
Gerhardt PD, Lindgren DL (1954) Penetration of packaging films: film materials used for food packaging tested for resistance to some common stored-product insects. Hilgardia 8:3–4
Riudavets J, Salas I, Pons MJ (2007) Damage characteristics produced by insect pests in packaging film. J Stored Prod Res 43:564–570
Miao SJ, Zhang YL (2010) Feeding and degradation effect on plastic of Zophobas morio. J Environ Entomol 32:435–444
Yang J, Yang Y, Wu W-M et al (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48:13776–13784. https://doi.org/10.1021/es504038a
Yang Y, Yang J, Wu W-M et al (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 1. chemical and physical characterization and isotopic tests. Environ Sci Technol 49:12080–12086. https://doi.org/10.1021/acs.est.5b02661
Yang Y, Yang J, Wu W-M et al (2015) Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 2. role of gut microorganisms. Environ Sci Technol 49:12087–12093. https://doi.org/10.1021/acs.est.5b02663
Bombelli P, Howe CJ, Bertocchini F (2017) Current biology polyethylene bio-degradation by caterpillars of the wax moth Galleria mellonella R292. Curr Biol 27:283–293
Billen P, Khalifa L, Van Gerven F et al (2020) Technological application potential of polyethylene and polystyrene biodegradation by macro-organisms such as mealworms and wax moth larvae. Sci Total Environ 735:139521. https://doi.org/10.1016/j.scitotenv.2020.139521
Cassone BJ, Grove HC, Elebute O et al (2020) Role of the intestinal microbiome in low-density polyethylene degradation by caterpillar larvae of the greater wax moth, Galleria mellonella. Proc R Soc B: Biol Sci 287:20200112. https://doi.org/10.1098/rspb.2020.0112
Kong HG, Kim HH, Chung J et al (2019) The Galleria mellonella hologenome supports microbiota-independent metabolism of long-chain hydrocarbon beeswax. Cell Rep 26:2451-2464.e5. https://doi.org/10.1016/j.celrep.2019.02.018
Ren L, Men L, Zhang Z et al (2019) Biodegradation of polyethylene by enterobacter sp D1 from the guts of wax moth Galleria mellonella. Int J Environ Res Public Health 16:1941. https://doi.org/10.3390/ijerph16111941
Kundungal H, Gangarapu M, Sarangapani S et al (2021) Role of pretreatment and evidence for the enhanced biodegradation and mineralization of low-density polyethylene films by greater waxworm. Environ Technol 42:717–730. https://doi.org/10.1080/09593330.2019.1643925
Lou Y, Ekaterina P, Yang SS et al (2020) Biodegradation of polyethylene and polystyrene by greater wax moth larvae (Galleria mellonella L.) and the effect of co-diet supplementation on the core gut microbiome. Environ Sci Technol 54:2821–2831. https://doi.org/10.1021/acs.est.9b07044
Sanluis-Verdes A, Colomer-Vidal P, Rodriguez-Ventura F et al (2022) Wax worm saliva and the enzymes therein are the key to polyethylene degradation by Galleria mellonella. Nat Commun 13:5568. https://doi.org/10.1038/s41467-022-33127-w
Peng B-Y, Su Y, Chen Z et al (2019) Biodegradation of polystyrene by dark (Tenebrio obscurus ) and yellow ( Tenebrio molitor ) mealworms (Coleoptera: Tenebrionidae). Environ Sci Technol 53:5256–5265. https://doi.org/10.1021/acs.est.8b06963
Wang Z, Xin X, Shi X, Zhang Y (2020) A polystyrene-degrading acinetobacter bacterium isolated from the larvae of Tribolium castaneum. Sci Total Environ 726:138564. https://doi.org/10.1016/j.scitotenv.2020.138564
Cucini C, Leo C, Vitale M et al (2020) Bacterial and fungal diversity in the gut of polystyrene-fed Alphitobius diaperinus (Insecta: Coleoptera). Animal Gene 17–18:200109. https://doi.org/10.1016/j.angen.2020.200109
Cucini C, Funari R, Mercati D et al (2022) Polystyrene shaping effect on the enriched bacterial community from the plastic-eating Alphitobius diaperinus (Insecta: Coleoptera). Symbiosis 86:305–313. https://doi.org/10.1007/s13199-022-00847-y
Woo S, Song I, Cha HJ (2020) Fast and Facile biodegradation of polystyrene by the gut microbial flora of Plesiophthalmus davidis Larvae. Appl Environ Microbiol. https://doi.org/10.1128/AEM.01361-20
Kundungal H, Gangarapu M, Sarangapani S et al (2019) Efficient biodegradation of polyethylene (HDPE) waste by the plastic-eating lesser waxworm (Achroia grisella). Environ Sci Pollut Res 26:18509–18519. https://doi.org/10.1007/s11356-019-05038-9
Suresh Kesti S, Chandrabanda Thimmappa S (2019) First report on biodegradation of low density polyethylene by rice moth larvae, Corcyra cephalonica (stainton). Holist Approach Environ 9:79–83. https://doi.org/10.33765/thate.9.4.2
Zhang Z, Peng H, Yang D et al (2022) Polyvinyl chloride degradation by a bacterium isolated from the gut of insect larvae. Nat Commun 13:5360. https://doi.org/10.1038/s41467-022-32903-y
Xian Z-N, Yin C-F, Zheng L et al (2023) Biodegradation of additive-free polypropylene by bacterial consortia enriched from the ocean and from the gut of Tenebrio molitor larvae. Sci Total Environ 892:164721. https://doi.org/10.1016/j.scitotenv.2023.164721
Xu Y, Xian Z-N, Yue W et al (2023) Degradation of polyvinyl chloride by a bacterial consortium enriched from the gut of Tenebrio molitor larvae. Chemosphere 318:137944. https://doi.org/10.1016/j.chemosphere.2023.137944
Bae J, Cho HW, Jung H et al (2021) Changes in intestinal microbiota due to the expanded polystyrene diet of mealworms (Tenebrio molitor). Indian J Microbiol 61:130–136. https://doi.org/10.1007/s12088-021-00922-w
Jiang S, Su T, Zhao J, Wang Z (2021) Biodegradation of polystyrene by Tenebrio molitor, Galleria mellonella, and Zophobas atratus larvae and comparison of their degradation effects. Polymers (Basel) 13:3539. https://doi.org/10.3390/polym13203539
Lou Y, Li Y, Lu B et al (2021) Response of the yellow mealworm (Tenebrio molitor) gut microbiome to diet shifts during polystyrene and polyethylene biodegradation. J Hazard Mater 416:126222. https://doi.org/10.1016/j.jhazmat.2021.126222
Yang L, Gao J, Liu Y et al (2021) Biodegradation of expanded polystyrene and low-density polyethylene foams in larvae of Tenebrio molitor Linnaeus (Coleoptera: Tenebrionidae): broad versus limited extent depolymerization and microbe-dependence versus independence. Chemosphere 262:127818. https://doi.org/10.1016/j.chemosphere.2020.127818
Luo L, Wang Y, Guo H et al (2021) Biodegradation of foam plastics by Zophobas atratus larvae (Coleoptera: Tenebrionidae) associated with changes of gut digestive enzymes activities and microbiome. Chemosphere 282:131006. https://doi.org/10.1016/j.chemosphere.2021.131006
Wang Y, Luo L, Li X et al (2022) Different plastics ingestion preferences and efficiencies of superworm (Fab.) and yellow mealworm (Tenebrio molitor Linn.) associated with distinct gut microbiome changes. Sci Total Environ 837:155719. https://doi.org/10.1016/j.scitotenv.2022.155719
Serrano-Antón B, Rodríguez-Ventura F, Colomer-Vidal P et al (2023) The virtual microbiome: a computational framework to evaluate microbiome analyses. PloS one 18:e0280391. https://doi.org/10.1371/journal.pone.0280391
Kwadha CA, Ongamo GO, Ndegwa PN et al (2017) The biology and control of the greater wax moth Galleria mellonella. Insects 8:61. https://doi.org/10.3390/insects8020061
Cassone BJ, Grove HC, Kurchaba N et al (2022) Fat on plastic: Metabolic consequences of an LDPE diet in the fat body of the greater wax moth larvae (Galleria mellonella). J Hazard Mater 425:127862. https://doi.org/10.1016/j.jhazmat.2021.127862
Latour S, Noel G, Serteyn L et al (2021) Multi-omics approach reveals new insights into the gut microbiome of Galleria mellonella (Lepidoptera:Pyralidae) exposed to polyethylene diet. bioRxiv. https://doi.org/10.1101/2021.06.04.446152
LeMoine CMR, Grove HC, Smith CM, Cassone BJ (2020) A very hungry caterpillar: polyethylene metabolism and lipid homeostasis in larvae of the greater wax moth (Galleria mellonella). Environ Sci Technol 54:14706–14715. https://doi.org/10.1021/acs.est.0c04386
Réjasse A, Waeytens J, Deniset-Besseau A et al (2022) Plastic biodegradation: Do Galleria mellonella larvae bioassimilate polyethylene? a spectral histology approach using isotopic labeling and infrared microspectroscopy. Environ Sci Technol 56:525–534. https://doi.org/10.1021/acs.est.1c03417
Ruiz Barrionuevo JM, Vilanova-Cuevas B, Alvarez A et al (2022) The bacterial and fungal gut microbiota of the greater wax moth, Galleria mellonella L. Consum Polyethyl Polystyr Front Microbiol 13:918861. https://doi.org/10.3389/fmicb.2022.918861
Ruiz Barrionuevo JM, Martín E, Galindo Cardona A et al (2022) Consumption of low-density polyethylene, polypropylene, and polystyrene materials by larvae of the greater wax moth, Galleria mellonella L. (Lepidoptera, Pyralidae), impacts on their ontogeny. Environ Sci Pollut Res 29:68132–68142. https://doi.org/10.1007/s11356-022-20534-1
Weber C, Pusch S, Opatz T (2017) Polyethylene bio-degradation by caterpillars? Curr Biol 27:R744–R745. https://doi.org/10.1016/j.cub.2017.07.004
Li W, Jin D, Shi C, Li F (2017) Midgut bacteria in deltamethrin-resistant, deltamethrin-susceptible, and field-caught populations of Plutella xylostella, and phenomics of the predominant midgut bacterium Enterococcus mundtii. Sci Rep 7:1947. https://doi.org/10.1038/s41598-017-02138-9
Vilanova C, Baixeras J, Latorre A, Porcar M (2016) The generalist inside the specialist: gut bacterial communities of two insect species feeding on toxic plants are dominated by Enterococcus sp. Front Microbiol 7:1005. https://doi.org/10.3389/fmicb.2016.01005
Tang X, Freitak D, Vogel H et al (2012) Complexity and variability of gut commensal microbiota in polyphagous lepidopteran larvae. PloS one 7:e36978. https://doi.org/10.1371/journal.pone.0036978
Fei C, Lu X, Qian Y et al (2006) Identification ofEnterococcus sp. from midgut of silkworm based on biochemical and 16S rDNA sequencing analysis. Ann Microbiol 56:201–205. https://doi.org/10.1007/BF03175006
Noël G, Serteyn L, Sare AR et al (2022) Co-diet supplementation of low density polyethylene and honeybee wax did not influence the core gut bacteria and associated enzymes of Galleria mellonella larvae (Lepidoptera: Pyralidae). Int Microbiol. https://doi.org/10.1007/s10123-022-00303-3
Nowak B, Pająk J, Drozd-Bratkowicz M, Rymarz G (2011) Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int Biodeterior Biodegradation 65:757–767. https://doi.org/10.1016/j.ibiod.2011.04.007
Peydaei A, Bagheri H, Gurevich L et al (2021) Mastication of polyolefins alters the microbial composition in Galleria mellonella. Environ Pollut 280:116877. https://doi.org/10.1016/j.envpol.2021.116877
Peydaei A, Bagheri H, Gurevich L et al (2020) Impact of polyethylene on salivary glands proteome in Galleria melonella. Comp Biochem Physiol Part D Genomics Proteomics 34:100678. https://doi.org/10.1016/j.cbd.2020.100678
Zhu P, Pan X, Li X et al (2021) Biodegradation of plastics from waste electrical and electronic equipment by greater wax moth larvae (Galleria mellonella). J Clean Prod 310:127346. https://doi.org/10.1016/j.jclepro.2021.127346
Wang S, Shi W, Huang Z et al (2022) Complete digestion/biodegradation of polystyrene microplastics by greater wax moth (Galleria mellonella) larvae: direct in vivo evidence, gut microbiota independence, and potential metabolic pathways. J Hazard Mater 423:127213. https://doi.org/10.1016/j.jhazmat.2021.127213
Pinchi JE, Ordoñez Gálvez JJ, Olivera CC, Benites-Alfaro E (2022) Environmental biotechnology: biodegradation of microplastics with larvae of Tenebrio Molitor and Galleria Mellonella. Chem Eng Trans 93:187–192
Rost-Roszkowska M, Piotrowska P, Chajec Ł et al (2022) Consumption of polypropylene by Galleria mellonella (Insecta, Lepidoptera, Pyralidae) larvae did not cause degenerative changes of internal organs. Environ Sci Pollut Res Int 29:68132–68142
Burd BS, Mussagy CU, de Lacorte SJ et al (2022) Galleria Mellonella larvae as an alternative to low-density polyethylene and polystyrene biodegradation. J Polym Environ 31:1232–1241. https://doi.org/10.1007/s10924-022-02696-8
Zhu P, Shen Y, Li X et al (2022) Feeding preference of insect larvae to waste electrical and electronic equipment plastics. Sci Total Environ 807:151037. https://doi.org/10.1016/j.scitotenv.2021.151037
Venegas S, Alarcon C, Gatica M et al (2023) Identification of Potential enzymes responsible of the biodegradation of polystyrene mediated by Galleria Mellonella. SSRN Electron J. https://doi.org/10.2139/ssrn.4340621
Nyamjav I, Jang Y, Lee YE, Lee S (2023) Physicochemical and structural evidence that Bacillus cereus isolated from the gut of waxworms (Galleria mellonella larvae) biodegrades polypropylene efficiently in vitro. J Polymers Environment 31:4274–4287
Barbehenn R V., Kristensen NP (2003) 6. Digestive and excretory systems. In: Band 4: Arthropoda, 2 Hälfte: Insecta, Lepidoptera, Moths and Butterflies, Teilband/Part 36, Vol 2: Morphology, Physiology, and Development. DE GRUYTER, pp 165–188
Hammer TJ, Janzen DH, Hallwachs W et al (2017) Caterpillars lack a resident gut microbiome. Proc Natl Acad Sci 114:9641–9646. https://doi.org/10.1073/pnas.1707186114
Hakkarainen M, Albertsson A-C (2004) Environmental Degradation of Polyethylene. In: Advances in Polymer Science. pp 177–200
Roy PK, Hakkarainen M, Varma IK, Albertsson AC (2011) Degradable polyethylene: fantasy or reality. Environ Sci Technol 45:4217–4227
Glazer L, Tom M, Weil S et al (2013) Hemocyanin with phenoloxidase activity in the chitin matrix of the crayfish gastrolith. J Exp Biol. https://doi.org/10.1242/jeb.080945
Besser K, Malyon GP, Eborall WS et al (2018) Hemocyanin facilitates lignocellulose digestion by wood-boring marine crustaceans. Nat Commun 9:5125. https://doi.org/10.1038/s41467-018-07575-2
Dey AS, Bose H, Mohapatra B, Sar P (2020) Biodegradation of unpretreated low-density polyethylene (LDPE) by Stenotrophomonas sp. and Achromobacter sp., isolated from waste dumpsite and drilling fluid. Front Microbiol 11:603210. https://doi.org/10.3389/fmicb.2020.603210
Amobonye A, Bhagwat P, Singh S, Pillai S (2021) Plastic biodegradation: frontline microbes and their enzymes. Sci Total Environ 759:143536. https://doi.org/10.1016/j.scitotenv.2020.143536
Bertocchini F, Arias CF (2023) Why have we not yet solved the challenge of plastic degradation by biological means? PloS Biol 21:e3001979. https://doi.org/10.1371/journal.pbio.3001979
Kumar S, Abedin MdM, Singh AK, Das S (2020) Role of Phenolic Compounds in Plant-Defensive Mechanisms. In: Plant Phenolics in Sustainable Agriculture. Springer Singapore, Singapore, pp 517–532
Ho BT, Roberts TK, Lucas S (2018) An overview on biodegradation of polystyrene and modified polystyrene: the microbial approach. Crit Rev Biotechnol 38:308–320. https://doi.org/10.1080/07388551.2017.1355293
Saastamoinen M, van der Sterren D, Vastenhout N et al (2010) Predictive adaptive responses: condition-dependent impact of adult nutrition and flight in the tropical butterfly Bicyclus anynana. Am Nat 176:686–698. https://doi.org/10.1086/657038
Huerta Lwanga E, Gertsen H, Gooren H et al (2016) Microplastics in the terrestrial ecosystem: implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ Sci Technol 50:2685–2691. https://doi.org/10.1021/acs.est.5b05478
Jâms IB, Windsor FM, Poudevigne-Durance T et al (2020) Estimating the size distribution of plastics ingested by animals. Nat Commun 11:1594. https://doi.org/10.1038/s41467-020-15406-6
Pitt JA, Trevisan R, Massarsky A et al (2018) Maternal transfer of nanoplastics to offspring in zebrafish (Danio rerio): a case study with nanopolystyrene. Sci Total Environ 643:324–334. https://doi.org/10.1016/j.scitotenv.2018.06.186
Rodriguez-Seijo A, Lourenço J, Rocha-Santos TAP et al (2017) Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ Pollut 220:495–503. https://doi.org/10.1016/j.envpol.2016.09.092
Anguissola S, Garry D, Salvati A et al (2014) High content analysis provides mechanistic insights on the pathways of toxicity induced by amine-modified polystyrene nanoparticles. PloS one 9:e108025. https://doi.org/10.1371/journal.pone.0108025
Funding
Open access funding provided by Università degli Studi della Basilicata within the CRUI-CARE Agreement. This work was supported by University of Basilicata and INPS within PhD Program Industry 4.0 and by the Roechling Foundation to F.B.
Author information
Authors and Affiliations
Contributions
Conceptualization: PF; Writing, original draft preparation: PF, AB; Writing, review and editing: PF, AB, CS, RS, CFA, RPP, FB; Supervision: PF.
Corresponding author
Ethics declarations
Conflict of interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Boschi, A., Scieuzo, C., Salvia, R. et al. Beyond Microbial Biodegradation: Plastic Degradation by Galleria mellonella. J Polym Environ 32, 2158–2177 (2024). https://doi.org/10.1007/s10924-023-03084-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-03084-6