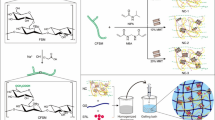
Abstract
Dual crosslinked interpenetrating polymer network (IPN) beads of sodium alginate reinforced with curdlan-grafted poly-N-isopropylacrylamide/montmorillonite [Cud-g-PNIPA-/MMT] nanocomposites were accomplished for effective delivery of erlotinib HCl (ERL) to triple-negative breast cancer (TNBC) cells. Different beads exhibited variable size (1.30–1.47 mm) and excellent drug entrapment efficiency (DEE, 49.35–93.01%), which were influenced by the contents of reinforcing clay (MMT) in the composites. The SEM photographs also displayed the spherical morphology of the beads, and the results of infrared, thermal, and X-ray diffraction studies conferred a compatible environment of ERL in the matrices. Moreover, the sustained drug release profiles (Q8h, 73.79–84.24%) of various matrices were best fitted in the Korsmeyer-Peppas kinetic model with Fickian diffusion-driven mechanism. Furthermore, the beads demonstrated temperature-responsive swelling behavior and their molar masses between crosslinks (\(\bar{M}\)c) estimated by Flory-Rehner equation were enhanced with temperature. The beads containing 20% MMT (F-3) displayed a faster biodegradability than the control matrices (F-1, 0% MMT). The mucin adsorption ability of F-1 and F-3 followed Freundlich isotherm and Langmuir isotherm, respectively. As compared to pristine drug, F-3 bestowed an increased sensitivity on MDA-MB-231 cells. Thus, the Cud/alginate IPN beads could be employed as efficient drug carriers for TNBC therapy.






Similar content being viewed by others
Data Availability
The authors declare that the data supporting the findings of this study are available within the paper.
References
André F, Zielinski CC (2012) Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann Oncol 23:vi46–vi51. https://doi.org/10.1093/annonc/mds195
Mirza Z, Karim S (2021) Nanoparticles-based drug delivery and gene therapy for breast cancer: recent advancements and future challenges. Sem Cancer Biol 69:226–237. https://doi.org/10.1016/j.semcancer.2019.10.020
Son S, Shin S, Rao NV, Um W, Jeon J, Ko H et al (2018) Anti-Trop2 antibody-conjugated bioreducible nanoparticles for targeted triple negative breast cancer therapy. Int J Biol Macromol 110:406–415. https://doi.org/10.1016/j.ijbiomac.2017.10.113
Pal SK, Childs BH, Pegram M (2011) Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat 125(3):627–636. https://doi.org/10.1007/s10549-010-1293-1
Wan X, Zheng X, Pang X, Pang Z, Zhao J, Zhang Z et al (2016) Lapatinib-loaded human serum albumin nanoparticles for the prevention and treatment of triple-negative breast cancer metastasis to the brain. Oncotarget 7(23):34038–34051. https://doi.org/10.18632/oncotarget.8697
Lin C, Ren Z, Yang X, Yang R, Chen Y, Liu Z et al (2020) Nerve growth factor (NGF)-TrkA axis in head and neck squamous cell carcinoma triggers EMT and confers resistance to the EGFR inhibitor erlotinib. Cancer Lett 472:81–96. https://doi.org/10.1016/j.canlet.2019.12.015
Bao B, Mitrea C, Wijesinghe P, Marchetti L, Girsch E, Farr RL et al (2017) Treating triple negative breast cancer cells with erlotinib plus a select antioxidant overcomes drug resistance by targeting cancer cell heterogeneity. Sci Rep 7(1):44125. https://doi.org/10.1038/srep44125
Varan G, Akkın S, Demirtürk N, Benito JM, Bilensoy E (2021) Erlotinib entrapped in cholesterol-depleting cyclodextrin nanoparticles shows improved antitumoral efficacy in 3D spheroid tumors of the lung and the liver. J Drug Target 29(4):439–453. https://doi.org/10.1080/1061186X.2020.1853743
Bera H, Abbasi YF, Gajbhiye V, Liew KF, Kumar P, Tambe P et al (2020) Carboxymethyl fenugreek galactomannan-g-poly(N-isopropylacrylamide-co-N,N′-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Mater Sci Eng: C 110:110628. https://doi.org/10.1016/j.msec.2020.110628
Yan J-K, Qiu W-Y, Wang Y-Y, Wu L-X, Cheung PCK (2018) Formation and characterization of polyelectrolyte complex synthesized by chitosan and carboxylic curdlan for 5-fluorouracil delivery. Int J Biol Macromol 107:397–405. https://doi.org/10.1016/j.ijbiomac.2017.09.004
Popescu I, Pelin IM, Suflet DM (2018) Dual-responsive hydrogels based on maleilated curdlan-graft-poly (N-isopropylacrylamide). Int J Polym Mater Polym Biomaterials 67(18):1069–1079. https://doi.org/10.1080/00914037.2017.1417289
Xu L, Liang X, You L, Yang Y, Fen G, Gao Y et al (2021) Temperature-sensitive poly (N-isopropylacrylamide)-chitosan hydrogel for fluorescence sensors in living cells and its antibacterial application. Int J Biol Macromol 189:316–323. https://doi.org/10.1016/j.ijbiomac.2021.08.057
Xu L, Zhong S, Gao Y, Cui X (2022) Thermo-responsive poly (N-isopropylacrylamide)-hyaluronic acid nano-hydrogel and its multiple applications. Int J Biol Macromol 194:811–818. https://doi.org/10.1016/j.ijbiomac.2021.11.133
Milster S, Chudoba R, Kanduč M, Dzubiella J (2019) Cross-linker effect on solute adsorption in swollen thermoresponsive polymer networks. Phys Chem Chem Phys 21(12):6588–6599. https://doi.org/10.1039/C8CP07601D
Bin Imran A, Esaki K, Gotoh H, Seki T, Ito K, Sakai Y et al (2014) Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat Commun 5(1):5124. https://doi.org/10.1038/ncomms6124
Liang L, Liu J, Gong XY (2000) Thermosensitive poly(N-isopropylacrylamide)-clay nanocomposites with enhanced temperature response. Langmuir 16:9895–9899. https://doi.org/10.1021/la000279v
Rodrigues LAdS, Figueiras A, Veiga F, de Freitas RM, Nunes LCC, da Silva Filho EC et al (2013) The systems containing clays and clay minerals from modified drug release: a review. Colloids Surf B 103:642–651. https://doi.org/10.1016/j.colsurfb.2012.10.068
Nayak AK, Hasnain MS, Aminabhavi TM (2021) Drug delivery using interpenetrating polymeric networks of natural polymers: a recent update. J Drug Deliv Sci Technol 66:102915. https://doi.org/10.1016/j.jddst.2021.102915
Ma H, Zhao J, Liu Y, Liu L, Yu J, Fan Y (2023) Controlled delivery of aspirin from nanocellulose-sodium alginate interpenetrating network hydrogels. Ind Crops Prod 192:116081. https://doi.org/10.1016/j.indcrop.2022.116081
Bulut E (2021) Development and optimization of Fe3+-crosslinked sodium alginate-methylcellulose semi-interpenetrating polymer network beads for controlled release of ibuprofen. Int J Biol Macromol 168:823–833. https://doi.org/10.1016/j.ijbiomac.2020.11.147
Truong DH, Le VKH, Pham TT, Dao AH, Pham TPD, Tran TH (2020) Delivery of erlotinib for enhanced cancer treatment: an update review on particulate systems. J Drug Deliv Sci Technol 55:101348. https://doi.org/10.1016/j.jddst.2019.101348
Bera H, Abbasi YF, Gajbhiye V, Ping LL, Salve R, Kumar P et al (2021) Chemosensitivity assessments of curdlan-doped smart nanocomposites containing erlotinib HCl. Int J Biol Macromol 181:169–179. https://doi.org/10.1016/j.ijbiomac.2021.03.152
Giri TK, Thakur D, Alexander A, Ajazuddin, Badwaik H, Tripathy M et al (2013) Biodegradable IPN hydrogel beads of pectin and grafted alginate for controlled delivery of diclofenac sodium. J Mater Sci Mater Med 24(5):1179–1190. https://doi.org/10.1007/s10856-013-4884-7
Pandey P, Dua K, Dureja H (2019) Erlotinib loaded chitosan nanoparticles: Formulation, physicochemical characterization and cytotoxic potential. Int J Biol Macromol 139:1304–1316. https://doi.org/10.1016/j.ijbiomac.2019.08.084
Erfani A, Flynn NH, Aichele CP, Ramsey JD (2020) Encapsulation and delivery of protein from within poly(sulfobetaine) hydrogel beads. J Appl Polym Sci 137(40):49550. https://doi.org/10.1002/app.49550
Kulkarni AR, Soppimath KS, Aminabhavi TM, Dave AM, Mehta MH (2000) Glutaraldehyde crosslinked sodium alginate beads containing liquid pesticide for soil application. J Controlled Release 63(1):97–105. https://doi.org/10.1016/S0168-3659(99)00176-5
Jing H, Huang X, Du X, Mo L, Ma C, Wang H (2022) Facile synthesis of pH-responsive sodium alginate/carboxymethyl chitosan hydrogel beads promoted by hydrogen bond. Carbohydr Polym 278:118993. https://doi.org/10.1016/j.carbpol.2021.118993
Wright L, Joyce P, Barnes TJ, Prestidge CA (2021) Mimicking the gastrointestinal mucus barrier: Laboratory-Based approaches to facilitate an enhanced understanding of mucus permeation. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.1c00814
Bera H, Abbasi YF, Yoke FF, Seng PM, Kakoti BB, Ahmmed SKM et al (2019) Ziprasidone-loaded arabic gum modified montmorillonite-tailor-made pectin based gastroretentive composites. Int J Biol Macromol 129:552–563. https://doi.org/10.1016/j.ijbiomac.2019.01.171
Hassanpour M, Jafari H, Sharifi S, Rezaie J, Lighvan ZM, Mahdavinia GR et al (2021) Salicylic acid-loaded chitosan nanoparticles (SA/CTS NPs) for breast cancer targeting: synthesis, characterization and controlled release kinetics. J Mol Struct 1245:131040. https://doi.org/10.1016/j.molstruc.2021.131040
Krey JF, Drummond M, Foster S, Porsov E, Vijayakumar S, Choi D et al (2016) Annexin A5 is the most abundant Membrane-Associated protein in Stereocilia but is dispensable for hair-bundle development and function. Sci Rep 6(1):27221. https://doi.org/10.1038/srep27221
Kabała-Dzik A, Rzepecka-Stojko A, Kubina R, Jastrzębska-Stojko Ż, Stojko R, Wojtyczka RD et al (2017) Migration rate inhibition of breast cancer cells treated by caffeic acid and caffeic acid phenethyl ester: an in vitro comparison study. Nutrients https://doi.org/10.3390/nu9101144
Shrikhande SS, Jain DS, Athawale RB, Bajaj AN, Goel P, Kamran Z et al (2015) Evaluation of anti-metastatic potential of Cisplatin polymeric nanocarriers on B16F10 melanoma cells. Saudi Pharm J 23(4):341–351. https://doi.org/10.1016/j.jsps.2014.08.004
Fukuhara G, Inoue Y (2010) Oligosaccharide sensing with chromophore-modified curdlan in aqueous media. Chem Commun 46(48):9128–9130. https://doi.org/10.1039/C0CC02568B
Kulkarni RV, Sa B (2008) Enteric delivery of ketoprofen through functionally modified poly(acrylamide-grafted-xanthan)-based pH-sensitive hydrogel beads: Preparation, in vitro and in vivo evaluation. J Drug Target 16(2):167–177. https://doi.org/10.1080/10611860701792399
Chanra J, Budianto E, Soegijono B (2019) Surface modification of montmorillonite by the use of organic cations via conventional ion exchange method. In: IOP conference series: materials science and engineering. IOP Publishing. p. 012057
Wu C, Peng S, Wen C, Wang X, Fan L, Deng R et al (2012) Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr Polym 89(2):497–503. https://doi.org/10.1016/j.carbpol.2012.03.034
Thambi T, Phan VH, Lee DS (2016) Stimuli-Sensitive Injectable Hydrogels based on polysaccharides and their Biomedical Applications. Macromol Rapid Commun 37(23):1881–1896. https://doi.org/10.1002/marc.201600371
Guo X, Kang J, Xu Z, Guo Q, Zhang L, Ning H et al (2021) Triple-helix polysaccharides: formation mechanisms and analytical methods. Carbohydr Polym 262:117962. https://doi.org/10.1016/j.carbpol.2021.117962
Pongjanyakul T, Rongthong T (2010) Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohydr Polym 81(2):409–419. https://doi.org/10.1016/j.carbpol.2010.02.038
Gomes RF, Lima LRM, Feitosa JPA, Paula HCB, de Paula RCM (2020) Influence of galactomannan molar mass on particle size galactomannan-grafted-poly-N-isopropylacrylamide copolymers. Int J Biol Macromol 156:446–453. https://doi.org/10.1016/j.ijbiomac.2020.04.004
Rampaka R, Ommi K, Chella N (2021) Role of solid lipid nanoparticles as drug delivery vehicles on the pharmacokinetic variability of Erlotinib HCl. J Drug Deliv Sci Technol 66:102886. https://doi.org/10.1016/j.jddst.2021.102886
Gontijo SML, Guimarães PPG, Viana CTR, Denadai ÂML, Gomes ADM, Campos PP et al (2015) Erlotinib/hydroxypropyl-β-cyclodextrin inclusion complex: characterization and in vitro and in vivo evaluation. J Incl Phenom Macrocyclic Chem 83(3):267–279. https://doi.org/10.1007/s10847-015-0562-3
Nyol S, Gupta M (2013) Immediate drug release dosage form: a review. J Drug Delivery Ther. https://doi.org/10.22270/jddt.v3i2.457
Ding H (2022) Modified-release Drug Products and Drug Devices. In: Ducharme MP, Shargel L (eds) Shargel and Yu’s Applied Biopharmaceutics and Pharmacokinetics, 8th edn. McGraw-Hill Education, New York
Yang W, Wiederhold NP, Williams RO (2008) Drug delivery strategies for improved azole antifungal action. Expert Opin Drug Deliv 5(11):1199–1216. https://doi.org/10.1517/17425240802457188
Sorasitthiyanukarn FN, Muangnoi C, Thaweesest W, Rojsitthisak P, Rojsitthisak P (2019) Enhanced cytotoxic, antioxidant and anti-inflammatory activities of curcumin diethyl disuccinate using chitosan-tripolyphosphate nanoparticles. J Drug Deliv Sci Technol 53:101118. https://doi.org/10.1016/j.jddst.2019.06.015
Tonnesen HH, Karlsen J (2002) Alginate in drug delivery systems. Drug Dev Ind Pharm 28(6):621–630. https://doi.org/10.1081/ddc-120003853
Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM (2013) Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence 7:419–434. https://doi.org/10.2147/PPA.S44646
Singh B, Sharma V, Chauhan D (2010) Gastroretentive floating sterculia–alginate beads for use in antiulcer drug delivery. Chem Eng Res Des 88(8):997–1012. https://doi.org/10.1016/j.cherd.2010.01.017
Bazban-Shotorbani S, Hasani-Sadrabadi MM, Karkhaneh A, Serpooshan V, Jacob KI, Moshaverinia A et al (2017) Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J Controlled Release 253:46–63. https://doi.org/10.1016/j.jconrel.2017.02.021
Fernando IPS, Lee W, Han EJ, Ahn G (2020) Alginate-based nanomaterials: fabrication techniques, properties, and applications. Chem Eng J 391:123823. https://doi.org/10.1016/j.cej.2019.123823
Wang D, Lv R, Ma X, Zou M, Wang W, Yan L et al (2018) Lysozyme immobilization on the calcium alginate film under sonication: development of an antimicrobial film. Food Hydrocolloids 83:1–8. https://doi.org/10.1016/j.foodhyd.2018.04.021
Vedadghavami A, Zhang C, Bajpayee AG (2020) Overcoming negatively charged tissue barriers: drug delivery using cationic peptides and proteins. Nano Today. https://doi.org/10.1016/j.nantod.2020.100898
Upadhyay M, Adena SKR, Vardhan H, Yadav SK, Mishra B (2019) Locust bean gum and sodium alginate based interpenetrating polymeric network microbeads encapsulating capecitabine: improved pharmacokinetics, cytotoxicity &in vivo antitumor activity. Mater Sci Eng C 104:109958. https://doi.org/10.1016/j.msec.2019.109958
Vaidya B, Parvathaneni V, Kulkarni NS, Shukla SK, Damon JK, Sarode A et al (2019) Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int J Biol Macromol 122:338–347. https://doi.org/10.1016/j.ijbiomac.2018.10.181
Wan X, Liu C, Lin Y, Fu J, Lu G, Lu Z (2019) pH sensitive peptide functionalized nanoparticles for co-delivery of erlotinib and DAPT to restrict the progress of triple negative breast cancer. Drug Delivery 26(1):470–480. https://doi.org/10.1080/10717544.2019.1576801
Funding
The present work received funding from fellowship of China Postdoctoral Science Foundation (grant no. 2021MD703857; grant recipient, Dr. Hriday Bera).
Author information
Authors and Affiliations
Contributions
Authors contributed equally.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bera, H., Abbasi, Y.F. & Thakur, A. Curdlan/Clay Nanocomposite-Reinforced Alginate Beads as Drug Carriers. J Polym Environ 32, 854–869 (2024). https://doi.org/10.1007/s10924-023-03036-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-03036-0