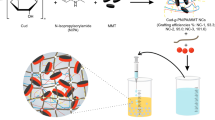
Abstract
Carboxymethyl fenugreek seed mucilage-grafted poly-N-isopropylacrylamide/montmorillonite [CFSM-g-PNIPA/MMT] nanocomposites (NCs) were dually crosslinked with gellan gum (GG) to afford interpenetrating polymer network (IPN) matrices for effective delivery of erlotinib HCl (ERL) to the triple-negative breast cancer (TNBC) cells. The IPN matrices with different reinforcing clay (MMT) contents (0–20%) demonstrated comparable size (1.64–1.80 mm) and acceptable drug loading capacity (DEE, 47.97–89.35%). The infrared, thermal and X-ray analyses implied the compatibility between drug and matrix constituents, and the SEM studies conferred the spherical morphology of the IPN matrices. Moreover, these hydrogel matrices portrayed temperature-responsive swelling profiles and their molar masses between crosslinks \(\left( {\bar{M}_{{\text{c}}} } \right)\) were increased with temperature. Among different matrices, the composites containing 20% MMT (F-3) revealed the sustained drug release profiles, which were best fitted to the Higuchi model with an anomalous transport-driven mechanism. These matrices (F-3) also exhibited pH-dependent swelling and drug release patterns. Furthermore, these matrices evidenced a slower biodegradability as compared to the reference composites (F-1,0% MMT). The mucin adsorption ability of matrices followed the Freundlich isotherm. The matrices (F-3) also displayed enhanced anti-proliferative and apoptosis-inducing potentials on TNBC cells relative to pure ERL. Thus, the GG/CFSM-based IPN matrices could be employed as efficient drug delivery vehicles for breast cancer therapy.





Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Nayak AK, Ansari MT, Sami F, Bera H, Hasnain MS (2019) Cashew gum in drug delivery applications. In: Hasnain MS, Nayak AK (eds) Natural polysaccharides in drug delivery and biomedical applications. Academic Press, London, pp 263–283
Nayak AK, Bera H (2019) In situ polysaccharide-based gels for topical drug delivery applications. In: Maiti S, Jana S (eds) Polysaccharide carriers for drug delivery. Woodhead Publishing, Duxford, pp 615–638
Bera H, Abbasi YF, Gajbhiye V, Liew KF, Kumar P, Tambe P et al (2020) Carboxymethyl fenugreek galactomannan-g-poly(N-isopropylacrylamide-co-N, N′-methylene-bis-acrylamide)-clay based pH/temperature-responsive nanocomposites as drug-carriers. Mater Sci Eng C 110:110628. https://doi.org/10.1016/j.msec.2020.110628
Bera H, Abbasi YF, Lee Ping L, Marbaniang D, Mazumder B, Kumar P et al (2020) Erlotinib-loaded carboxymethyl temarind gum semi-interpenetrating nanocomposites. Carbohydr Polym 230:115664. https://doi.org/10.1016/j.carbpol.2019.115664
Nayak AK, Pal D, Das S (2013) Calcium pectinate-fenugreek seed mucilage mucoadhesive beads for controlled delivery of metformin HCl. Carbohydr Polym 96(1):349–357. https://doi.org/10.1016/j.carbpol.2013.03.088
Nayak AK, Pal D, Santra K (2014) Development of calcium pectinate-tamarind seed polysaccharide mucoadhesive beads containing metformin HCl. Carbohydr Polym 101:220–230. https://doi.org/10.1016/j.carbpol.2013.09.024
Bera H, Abbasi YF, Yoke FF, Seng PM, Kakoti BB, Ahmmed SKM, Bhatnagar P (2019) Ziprasidone-loaded arabic gum modified montmorillonite-tailor-made pectin based gastroretentive composites. Int J Biol Macromol 129:552–563. https://doi.org/10.1016/j.ijbiomac.2019.01.171
del Real A, Wallander D, Maciel A, Cedillo G, Loza H (2015) Graft copolymerization of ethyl acrylate onto tamarind kernel powder, and evaluation of its biodegradability. Carbohydr Polym 117:11–18. https://doi.org/10.1016/j.carbpol.2014.09.044
Nagaraja K, Rao KM, Rao KSVK, Han SS (2022) Dual responsive tamarind gum-co-poly(N-isopropyl acrylamide-co-ethylene glycol vinyl ether) hydrogel: a promising device for colon specific anti-cancer drug delivery. Colloids Surf A Physicochem Eng 641:128456. https://doi.org/10.1016/j.colsurfa.2022.128456
Ma J, Zhang L, Fan B, Xu Y, Liang B (2008) A novel sodium carboxymethylcellulose/poly(N-isopropylacrylamide)/Clay semi-IPN nanocomposite hydrogel with improved response rate and mechanical properties. J Polym Sci B Polym Phys 46(15):1546–1555. https://doi.org/10.1002/polb.21490
Wilpiszewska K, Antosik AK, Spychaj T (2015) Novel hydrophilic carboxymethyl starch/montmorillonite nanocomposite films. Carbohydr Polym 128:82–89. https://doi.org/10.1016/j.carbpol.2015.04.023
Bera H, Abbasi YF, Thakur A (2023) Curdlan/clay nanocomposite-reinforced alginate beads as drug carriers. J Polym Environ. https://doi.org/10.1007/s10924-023-03036-0
Ramezanian S, Moghaddas J, Roghani-Mamaqani H, Rezamand A (2023) Dual pH- and temperature-responsive poly(dimethylaminoethyl methacrylate)-coated mesoporous silica nanoparticles as a smart drug delivery system. Sci Rep 13(1):20194. https://doi.org/10.1038/s41598-023-47026-7
Bera H, Abbasi YF, Gajbhiye V, Ping LL, Salve R, Kumar P et al (2021) Chemosensitivity assessments of curdlan-doped smart nanocomposites containing erlotinib HCl. Int J Biol Macromol 181:169–179. https://doi.org/10.1016/j.ijbiomac.2021.03.152
Ghorbani M, Nezhad-Mokhtari P, Mahmoodzadeh F (2021) Incorporation of oxidized pectin to reinforce collagen/konjac glucomannan hydrogels designed for tissue engineering applications. Macromol Res 29(4):289–296
Erfani A, Flynn NH, Aichele CP, Ramsey JD (2020) Encapsulation and delivery of protein from within poly (sulfobetaine) hydrogel beads. J Appl Polym Sci 137(40):49550. https://doi.org/10.1002/app.49550
Wright L, Joyce P, Barnes TJ, Prestidge CA (2021) Mimicking the gastrointestinal mucus barrier: laboratory-based approaches to facilitate an enhanced understanding of mucus permeation. ACS Biomater Sci Eng. https://doi.org/10.1021/acsbiomaterials.1c00814
Kevadiya BD, Rajkumar S, Bajaj HC, Chettiar SS, Gosai K, Brahmbhatt H et al (2014) Biodegradable gelatin–ciprofloxacin–montmorillonite composite hydrogels for controlled drug release and wound dressing application. Colloids Surf B 122:175–183. https://doi.org/10.1016/j.colsurfb.2014.06.051
Gerlier D, Thomasset N (1986) Use of MTT colorimetric assay to measure cell activation. J Immunol Methods 94(1):57–63. https://doi.org/10.1016/0022-1759(86)90215-2
Krey JF, Drummond M, Foster S, Porsov E, Vijayakumar S, Choi D et al (2016) Annexin A5 is the most abundant membrane-associated protein in stereocilia but is dispensable for hair-bundle development and function. Sci Rep 6(1):27221. https://doi.org/10.1038/srep27221
Chakka VP, Zhou T (2020) Carboxymethylation of polysaccharides: synthesis and bioactivities. Int J Biol Macromol 165:2425–2431. https://doi.org/10.1016/j.ijbiomac.2020.10.178
Ganguly S, Maity T, Mondal S, Das P, Das NC (2017) Starch functionalized biodegradable semi-IPN as a pH-tunable controlled release platform for memantine. Int J Biol Macromol 95:185–198. https://doi.org/10.1016/j.ijbiomac.2016.11.055
Guo H, de Magalhaes GM, Ducouret G, Hourdet D (2018) Cold and hot gelling of alginate-graft-PNIPAM: a schizophrenic behavior induced by potassium salts. Biomacromol 19(2):576–587. https://doi.org/10.1021/acs.biomac.7b01667
Wu C, Peng S, Wen C, Wang X, Fan L, Deng R, Pang J (2012) Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr Polym 89(2):497–503. https://doi.org/10.1016/j.carbpol.2012.03.034
Huang X, Ge M, Wang H, Liang H, Meng N, Zhou N (2022) Functional modification of polydimethylsiloxane nanocomposite with silver nanoparticles-based montmorillonite for antibacterial applications. Colloids Surf A Physicochem Eng 642:128666. https://doi.org/10.1016/j.colsurfa.2022.128666
Yang F, Xia S, Tan C, Zhang X (2013) Preparation and evaluation of chitosan-calcium-gellan gum beads for controlled release of protein. Eur Food Res Technol 237(4):467–479. https://doi.org/10.1007/s00217-013-2021-y
Bulut E (2021) Development and optimization of Fe3+-crosslinked sodium alginate-methylcellulose semi-interpenetrating polymer network beads for controlled release of ibuprofen. Int J Biol Macromol 168:823–833. https://doi.org/10.1016/j.ijbiomac.2020.11.147
Pongjanyakul T, Rongthong T (2010) Enhanced entrapment efficiency and modulated drug release of alginate beads loaded with drug–clay intercalated complexes as microreservoirs. Carbohydr Polym 81(2):409–419. https://doi.org/10.1016/j.carbpol.2010.02.038
Gomes RF, Lima LRM, Feitosa JPA, Paula HCB, de Paula RCM (2020) Influence of galactomannan molar mass on particle size galactomannan-grafted-poly-N-isopropylacrylamide copolymers. Int J Biol Macromol 156:446–453. https://doi.org/10.1016/j.ijbiomac.2020.04.004
Rampaka R, Ommi K, Chella N (2021) Role of solid lipid nanoparticles as drug delivery vehicles on the pharmacokinetic variability of erlotinib HCl. J Drug Deliv Sci Technol 66:102886. https://doi.org/10.1016/j.jddst.2021.102886
Gontijo SML, Guimarães PPG, Viana CTR, Denadai ÂML, Gomes ADM, Campos PP et al (2015) Erlotinib/hydroxypropyl-β-cyclodextrin inclusion complex: characterization and in vitro and in vivo evaluation. J Incl Phenom Macrocycl Chem 83(3):267–279. https://doi.org/10.1007/s10847-015-0562-3
Yadav H, Agrawal R, Panday A, Patel J, Maiti S (2022) Polysaccharide-silicate composite hydrogels: review on synthesis and drug delivery credentials. J Drug Deliv Sci Technol 74:103573. https://doi.org/10.1016/j.jddst.2022.103573
Budha NR, Frymoyer A, Smelick GS, Jin JY, Yago MR, Dresser MJ et al (2012) Drug absorption interactions between oral targeted anticancer agents and PPIs: Is pH-dependent solubility the Achilles heel of targeted therapy? Clin Pharmacol Ther 92(2):203–213. https://doi.org/10.1038/clpt.2012.73
Park JH, Shin HJ, Kim MH, Kim JS, Kang N, Lee JY et al (2016) Application of montmorillonite in bentonite as a pharmaceutical excipient in drug delivery systems. J Pharm Investig 46(4):363–375. https://doi.org/10.1007/s40005-016-0258-8
Singh B, Sharma V, Chauhan D (2010) Gastroretentive floating sterculia–alginate beads for use in antiulcer drug delivery. Chem Eng Res Des 88(8):997–1012. https://doi.org/10.1016/j.cherd.2010.01.017
Bazban-Shotorbani S, Hasani-Sadrabadi MM, Karkhaneh A, Serpooshan V, Jacob KI, Moshaverinia A, Mahmoudi M (2017) Revisiting structure-property relationship of pH-responsive polymers for drug delivery applications. J Control Release 253:46–63. https://doi.org/10.1016/j.jconrel.2017.02.021
Fernando IPS, Lee W, Han EJ, Ahn G (2020) Alginate-based nanomaterials: fabrication techniques, properties, and applications. J Chem Eng 391:123823. https://doi.org/10.1016/j.cej.2019.123823
Pavani P, Kumar K, Rani A, Venkatesu P, Lee M-J (2021) The influence of sodium phosphate buffer on the stability of various proteins: insights into protein-buffer interactions. J Mol Liq 331:115753. https://doi.org/10.1016/j.molliq.2021.115753
Wang D, Lv R, Ma X, Zou M, Wang W, Yan L et al (2018) Lysozyme immobilization on the calcium alginate film under sonication: development of an antimicrobial film. Food Hydrocoll 83:1–8. https://doi.org/10.1016/j.foodhyd.2018.04.021
Sosnik A, Das Neves J, Sarmento B (2014) Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog Polym Sci 39(12):2030–2075. https://doi.org/10.1016/j.progpolymsci.2014.07.010
García-Guzmán P, Medina-Torres L, Calderas F, Bernad-Bernad MJ, Gracia-Mora J, Marcos X et al (2019) Rheological mucoadhesion and cytotoxicity of montmorillonite clay mineral/hybrid microparticles biocomposite. Appl Clay Sci 180:105202. https://doi.org/10.1016/j.clay.2019.105202
Sorasitthiyanukarn FN, Muangnoi C, Thaweesest W, Rojsitthisak P, Rojsitthisak P (2019) Enhanced cytotoxic, antioxidant and anti-inflammatory activities of curcumin diethyl disuccinate using chitosan-tripolyphosphate nanoparticles. J Drug Deliv Sci Technol 53:101118. https://doi.org/10.1016/j.jddst.2019.06.015
Yamasaki F, Zhang D, Bartholomeusz C, Sudo T, Hortobagyi GN, Kurisu K, Ueno NT (2007) Sensitivity of breast cancer cells to erlotinib depends on cyclin-dependent kinase 2 activity. Mol Cancer Ther 6(8):2168–2177. https://doi.org/10.1158/1535-7163.Mct-06-0514
Upadhyay M, Adena SKR, Vardhan H, Yadav SK, Mishra B (2019) Locust bean gum and sodium alginate based interpenetrating polymeric network microbeads encapsulating capecitabine: improved pharmacokinetics, cytotoxicity & in vivo antitumor activity. Mater Sci Eng C 104:109958. https://doi.org/10.1016/j.msec.2019.109958
Vaidya B, Parvathaneni V, Kulkarni NS, Shukla SK, Damon JK, Sarode A et al (2019) Cyclodextrin modified erlotinib loaded PLGA nanoparticles for improved therapeutic efficacy against non-small cell lung cancer. Int J Biol Macromol 122:338–347. https://doi.org/10.1016/j.ijbiomac.2018.10.181
Funding
The present work was supported by the fellowship of China Postdoctoral Science Foundation (grant no. 2021MD703857; grant recipient, Dr. Hriday Bera).
Author information
Authors and Affiliations
Contributions
YFA contributed to preparation and characterization of drug-loaded formulations, writing, reviewing and editing; HB contributed to conceptualization, characterization of drug-loaded formulations, supervision, writing, reviewing and editing; AT involved in cell culture experiments of various scaffolds; YFA and HB contributed equally.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Abbasi, Y.F., Bera, H. & Thakur, A. Erlotinib-entrapped gellan gum/carboxymethyl fenugreek seed mucilage-based thermo/pH-sensitive matrices. Polym. Bull. (2024). https://doi.org/10.1007/s00289-024-05240-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00289-024-05240-x