Abstract
Production of bacterial cellulose hydrogel and its evaluation as a proton exchange membrane (PEM) was evaluated. Initially, the bacterial cellulose hydrogel membranes (BCH) was produced by fermentation in a 600 mL bioreactor with a 300 mL medium volume, 10% v/v inoculum with Komagataeibacter hansenii under static conditions, and a temperature of 30 °C. The bacteria were cultivated in Hestrin-Schramm (HS) medium with pH adjustment to 6.6 with HCl and/or NaOH. Five culture media were evaluated to obtain uniformity on the surface and a rapid formation of BCH membrane: HS (M1), M1 + green tea extract (M3), M1 + mixture of extra thyme and green tea (M4), and M1 + glycerin (M5). The kinetics of BCH production was followed by digital images. Subsequently, BCH production cellulose was carried out using M5 under the same operating conditions. After 3, 5, 10 and 13 days of fermentation, the thickness of BCH formed was measured, respectively, as 0.301 ± 0.008 cm, 0.552 ± 0.026 cm, 0.584 ± 0.03 cm and 0.591 ± 0.018 cm. Finally, BCH was characterized by porosity, water absorption capacity, ion exchange capacity, mechanical strength and diffusivity. The results showed that thinner membranes favor the processes of ion exchange (0.143 H+mmol g−1) and water absorption (93%). On the other hand, thicker membranes enhance physical parameters of transport across the membrane and its operability. Nevertheless, BCH membranes can be a good alternative as PEM to microbial fuel cell once they are functionalized.
Similar content being viewed by others
Introduction
Global warming, atmospheric pollution, added to the depletion of fossil fuels has promoted renewable energy production technologies [33]. This corresponds to a world market valued at US$ 881 billion in 2020 that may reach US$ 1,977 billion in 2030 [7].
A renewable energy approach may employ proton exchange membrane fuel cells (PEMFCs) [7, 55], in particular microbial fuel cells (MFCs) [38]. PEMFCs have an estimated market of US$2.10 billion in 2021 up to US$22.74 billion in 2028 [11]. In this technology, in a typical configuration, two-chambered MFC has the anode and cathode compartments separated by a proton exchange membrane (PEM), the anodic and cathodic electrodes are connected by a wire, and electrons from the oxidation reactions in the anode compartment are passed through the wire to the cathode, where they are normally combined with oxygen and protons to form water [44].
The efficiency of an MFC is influenced by several factors, such as oxygen supply and consumption in the cathodic chamber, oxidation of organic substrate in the anodic chamber, electron transfer from the anodic chamber to the electrode surface, and cation transfer across the exchange membrane between the anode and the cathode [2].This can increase the pH gradient and, therefore, the internal resistance of the cell, although it is essential for increasing coulombic efficiency [25].
Despite decades of development, PEM technology still lack wide acceptance in the market, especially in vehicular transport systems [61]. The cost of fuel cells needs to be reduced by over 40 to turn possible of global commercialization of PEM [59]. The challenges for the market for this energy vector [21] are concentrated in the costs of ion exchange membranes [2, 22, 25], and suitable catalyst for the fuel cells [21].
Nafion is the most used membrane in PEM applications due to its high proton conductivity, good chemical stability, excellent mechanical properties and low internal resistance [18]. It is a fluoropolymer-copolymer based on sulfonated tetrafluoroethylene discovered in the late 1960s by DuPont. Despite its widespread use, its costs and its non-biodegradable nature, as well as its biofouling [41] has led to the search for alternative and innovative membranes for use in MFC. Thus, the research focus is to reduce the costs of these components, so that energy on an industrial scale can be introduced in the markets [15, 62].
Polymer membranes are the “heart” of fuel cells and therefore chemists are investigating new strategies to synthesize materials suitable for their production [59]. An alternative is the search for ecological materials to replace synthetic materials, with a view to the sustainability of the processes [45]. Therefore, the use of bio-based materials as substrates for the fabrication of key fuel cell components, to minimize the impact of their production, although incipient, is growing exponentially. Indeed, natural polymers such as cellulose and their nanoscale forms, cellulose nanocrystals, cellulosic nanofibrils and bacterial nanocellulose are suitable materials for engineering two main components of PEM cells. Despite intense R&D activity, little has been reviewed on the potential application of three forms of cellulose with less than one dimension at the nanoscale in the field of fuel cells [58].
Studies show that bacterial cellulose is a versatile renewable biomaterial that can be used as a hydrophilic matrix for incorporation of metals into thin, flexible and thermally stable membranes. Unlike plant cellulose, bacterial cellulose (BCH) catalyzes the deposition of metals within its structure to generate a finely divided homogeneous catalyst layer [9].
BCH is a type of cellulose that can be produced from bacteria such as Aerobacter, Alcaligenes and Achromobacter [32, 63]. Its characteristics are good mechanical properties, low density, large surface area, high porosity, non-toxicity, sustainable regeneration [32], high water absorption, high chemical purity, high crystallinity, good biocompatibility and biodegradability [29], easy growth on various substrates, in addition to controllable shape and texture [6, 14, 28, 54]. BCH can be applied in the form of hydrogels [19], sponges, films and capsules for many applications including food packaging, sensors, flame retardants, water treatment, wound dressings [32, 45], dielectric and magnetic components [20]. In addition, applications are found in the areas of medicine, nanomaterials, functional foods, among others [28, 54].
BCH has aroused several interests in the industry, although most studies focus on its properties for biomedicine. It is possible to use BCH directly as a polymer matrix with ex-situ and in-situ modifications to adapt the functional properties of the material to its purpose [4].
Thus, in this research, bacterial cellulose hydrogel membrane in static culture is produced using different culture media, and its performance as a proton exchange membrane is subsequently evaluated.
Materials and Methods
Culture Conditions and Synthesis of BCH Membranes
Bacterial cellulose hydrogel membranes (BCH) were produced in a 600 mL bioreactor with an effective volume of 300 mL. Previously, the bioreactor was sanitized with 15% v/v sodium hypochlorite for 35 min. The fermentation was inoculated with 10% v/v Komagataeibacter hansenii under static conditions and at 30 °C. The bacterium was cultivated for 13 days in 5 modified media using Hestrin-Schramm (HS) as a base, summarized in Table 1 [6, 8, 14], with pH adjustment to 6.6 with HCl and/or NaOH.
HS medium containing glucose 20 g/L, yeast extract g/L, peptone 5 g/L, citric acid 1.15 g/L, Na2HPO4 2.7 g/L. Thyme and green tea infusion were used as supplements and prepared using 0.6 g of each in 50 mL of water at 90 °C for 30 min. Subsequently, they were filtered and incorporated into the culture medium.
After 3, 5, 10, and 13 days of fermentation, samples of BCH membranes were extracted, from the culture medium, for purification and further characterization. Figure 1 illustrates the production of BCH membranes.
Following cultivation, BCH pellicles were harvested and rinsed in deionized water. Next, BCH membranes were treated with 0.1 M NaOH at 80 °C in a water bath (3 ×) and then again rinsed in deionized water to remove residual NaOH. Purified BCH membranes was then stored at 4 °C for further analysis [6, 63].
BCH membranes thickness was determined from digital images (Image J software), according to the methodology used by Guilherme et al. [16]. Initially, the image scale was calibrated and later a line was drawn, from 10 different sections of the membrane, to determine the average thickness and decrease the measurement error [16].
BCH membranes, to the best culture media, who showed uniformity on the surface and a rapid formation of membrane were characterized.
Characterization Of BCH Membranes
Ion Exchange Capacity (IEC)
The IEC values of BCH were measured with the titration method according to a previous study [63]. The BCH membranes were soaked in a 1.0 M NaCl solution for 24 h at ambient temperature to ensure the proton were replaced completely by the sodium ion. This solution was subsequently titrated against a 0.005 M sodium hydroxide solution to neutralize the exchanged protons using phenolphthalein as an indicator. After that, the calculated IEC value was obtained through Eq. (1).
where IEC is the ion exchange capacity (mmol g−1); a is the added titrant volume at the equivalent point (mL); b is the molar concentration of the titrant; m is the dry membrane weight (g).
Water Absorption Capacity
BCH membranes samples were removed from storage, the surface water was gently removed using paper filter, and then the wet mass was determined using an analytical balance. Next, BCH membranes samples were dried overnight at 50 °C in an oven, to completely remove water and then weighted again. The moisture content percentage (W%) was calculated using Eq. (2) [28].
where: Wwet is the wet mass of BCH in g, Wdry is the dry mass of BCH in g.
Porosity
The porosity of BCH membranes was determined by the liquid displacement method [52].The dimensions of samples were measured, and their volumes were calculated. The samples were weighed and soaked in water at room temperature. After immersion for 24 h, the samples were wiped by a filter paper and weighed. The porosity of three samples was calculated as Eq. (3):
where mi is the initial dry weight of each sample, mf, is the weight of each sample after immersion, ρ represents the density of water and VMTP is the volume of each sample.
Test of Mechanical Properties
The mechanical performance, maximum breaking stress, and the modulus of elasticity of BCH membranes, were recorded using a texture measuring device (TA, XT-Plus) with a loading velocity of 5 mm/min. Prior to the examination, samples were cut into 5 × 1 cm pieces. Each group was tested three times, and the mean value was determined [63].
Proton Conductivity
Proton conductivity was measured by an AC impedance technique using an electrochemical impedance analyzer (Metrohm AUTOLAB, PGSTAT204) where the AC frequency was scanned from 100,000 Hz to 0.1 Hz at voltage amplitude of 100 mV [26]. The BCH with a diameter of 1.5 cm and thickness of ~ 0.301 cm and Nafion® 117 were sandwiched in a Teflon conductivity cell equipped with graphite foil contacts on opposite sides of the membrane. The proton conductivity ( σ) (S cm−1) was calculated according to the following Eq. (4):
where L is the thickness of membrane, R is the membrane resistance derived from the intercept of the high-frequency impedance with the real axis, A is the area of the membrane. The experiment was conducted in a room at 25° C.
Diffusion Coefficients Determination
The mass transfer coefficients of oxygen and Ca2+ (DO e DCa, respectively) through the raw BCH membrane were determined using abiotic cells [27]. A conductivity probe (DDS-11A, LIDA Instrument Co., China) was placed in the chamber filled with water. Both chambers were mixed intensively with magnetic stirring. The mass transfer coefficient of Ca2+ through the membrane was determined by monitoring the conductivity over time, which can be translated into Ca2+ concentration using a standard curve of concentration vs conductivity. In the case of DO, a dissolved oxygen (DO) probe (WTW) was placed in the anodic chamber as well as the cathodic chamber that was filled with water saturated with O2 and continuously mixed to maintain saturated DO conditions. DO was determined by monitoring the concentration of DO over time and using mass balances Eq. (5) [64].
where Di (m2/s) is the mass transfer coefficients of i specie; Vo is the liquid volume in each chamber; Lo is the membrane thickness; Ao is the cross-sectional area of the membrane; x1;0 is the initial concentration of Ca2+ in the cathodic chamber; x2;t is the Ca2+ concentrations in the chamber filled with water at time t. The diffusion coefficients for oxygen could be determined in the same way.
Results and Discussion
BCH Production
The BCH is excreted as exopolysaccharide by aerobic bacteria, such as Achromobacter, Alcaligenes, Aerobacter, Agrobacterium, Azotobacter, Komagataeibacter (formerly Gluconacetobacter), Pseudomonas, Rhizobium, Sarcina, Dickeya and Rhodobacter [4]. In the present study, bacterial cellulose was produced from Komagataeibacter hansenii as they are effective bacteria for BCH production according to studies carried out by [14].
Under a static culture condition, the acetic acid bacteria extrude a cellulose nanofiber in random directions, and thereby a 3D network structure termed “pellicle” is to be formed [54]. K. hansenii are aerobic bacteria, which colonize the oxygen-rich air/liquid interface of the spatially structured microcosm and metabolize sugar to produce a thick cellulosic film on their surface [51].
Likewise, bacteria, when propagated in static liquid culture, grow in the liquid phase, adhere to the film, and remain at the air–liquid interface starting as a thin film that first coats the surface and then thickens over time. The biosynthesized surface film generated by the bacteria adheres tightly to the cell walls of the microorganism and gradually floats to the bottom as fermentation proceeds [51].
In the present study, static culture was used to create biofilms and Hestrin-Schramm (HS) medium as the initial medium, as it is a standard and more appropriate medium for cultivation. In order to scale the process, the system was sanitized and not sterilized. In addition, the modification of the culture medium with thyme was used, which has a broad spectrum of fungicidal activity [48], in addition to green tea [24] and glycerin [47], which is an oily source of carbon.
Green tea was added to the culture medium due to its content of polyphenols, flavonoids, proteins, and amino acids [60]. Its content increases the acidity content, which indicates the consumption of the carbon source and the development of acetic acid-producing bacteria [56]. Furthermore, studies with green tea leaves observed a conversion yield of 0.32 and 0.31 g of bacterial nanocellulose per g of sugar. Therefore, tea leaves are also considered a raw material option for high yields with low production costs [30]. The caffeine content in a green tea beverage was estimated to be 0.039 mg/mL. When the tea concentration increases, the cellulose yield decreases, as the increase in polyphenol is an inhibitor of biofilm production by Gram negative bacteria [49].
The results of the growth of bacterial cellulose in the different culture media are presented in Fig. 2.
Sanitation of the system was effective; the culture media were not contaminated, and BCH was developed in each of them. Thus, the thermal process is reduced, making it possible to lower the costs on a future scale. The highest values for BCH membranes thickness growth were when M4 and M5 culture media were used, reaching values of 0.291 ± 0.008 cm and 0.301 ± 0.008 cm on day 3, 0.405 ± 0.018 cm and 0.552 ± 0.026 cm on day 5, 0.491 ± 0.078 cm and 0.584 ± 0.038 cm on day 10 and 0.581 ± 0.022 cm and 0.591 ± 0.018 cm on day 13, respectively.
The M5 medium was selected to continue the process, due to uniformity on the surface of CB, which was not observed for the M4 medium. It is believed that glycerin may have contributed to the uniformity of the cellulosic film formed on the surface, as it inhibits the growth of fungi and cellulase production that can impair on manufacturing of BCH membranes. Glycerol helps to slow the process of dehydration, past studies reported than BCH with have a much stronger consistency compared BCH who growth in another cultures media without glycerol. [3, 30, 46].
Figure 3 shows the behavior of the BCH membrane formed using the M4 and M5 culture media.
BCH membranes were obtained from day 3 to day 13. Studies carried out by Laavanya et al. [30] show that a period of 7 to 15 days is adequate to obtain good BCH membranes.
Effect of Thickness on Physical and Chemical Properties
In this study, BCH from M5 with thicknesses of 0.591 ± 0.018 cm were produced on day 13, a value approximately ten times greater than the results of the study carried out by Costa et al. [8] by fermentation at 30 °C for 10 days. These authors achieved a thickness of 0.062 ± 0.08 cm with a 100 mL culture medium in 500 mL bioreactors.
Structurally, BCH is thinner and less branched compared to plant cellulose, which gives BCH properties such as a large surface area, greater water absorption, and better mechanical strength in the wet state [30].
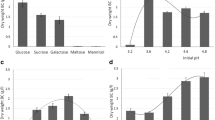
The BCH produced in the M5 culture medium was characterized and the results are shown in Fig. 4. The ion exchange capacity (IEC) of BCH was determined, as it is a relevant property in fuel cell membranes. It is important to highlight the fact that ion exchange capacity values are associated with the proton conductivity and membrane electric resistance. Thus, a membrane with higher IEC showed a higher proton conductivity as well as energy production [50].
As it can be observed in Fig. 4A, IEC is substantially enhanced with increasing BCH thickness, and values ranging between 0.082 and 0.143 H+mmol g−1. The maximum test thickness differs only by 10% from the IEC of the smallest.
This must be credited to the presence of large amounts of –OH groups on the surface of neat BCH [31]. The hydroxyl groups have an affinity toward hydrogen ions and could form bridges. Therefore, the higher thickness of BCH might have made the membrane more acceptable to H+ ion and exchanged by Na+ ion.
The IEC values of pure BCH are very close to zero [13], that is, they are low in relation to other membranes used in fuel cells, but functionalization or doping with organic or inorganic acids such as H3PO4, pigments, or other substances can improve this property [26]. In tests carried out by Gadim et al. [12], BCH was functionalized with poly(4-styrene sulfonic acid) showed and an increase in the IEC to 2.25 mmol g−1. In another work, BCH was functionalized with LiCl and reached an IEC of 2.57 mmol g−1, indicating that when functionalizing BCH, the IEC increases [23]. The chemical structure of BCH and the presence of hydroxyl groups on the surface allow functionalization or doping with additives to improve its physical, chemical and mechanical properties [30].
The behavior of BCH in terms of water absorption is very similar in all thicknesses (Fig. 4B). The magnitude of water uptake for BCH membranes was between 93 and 68%, for 0.301 cm and 0.531 cm of thickness, respectively. Proton diffusion from the anode chamber to the cathode compartment is indispensable for MFC to perform efficiently. One type of mechanism of proton transport is vehicle diffusion via protonated water molecules in the form of H3O+ or H9O4+ which contributes about 22% of the total proton passage [41]. Therefore, the magnitude of water uptake through absorption by the exchange membrane is critical in determining its proton conductivity [53]. Some studies report water uptakes of BCH pure in the range of 30–150% [10, 17] due to the hydrophilic nature of BCH. However, this property depends on the culture medium in which BCH was developed because this is the basis of its structure.
The applications of BCH are effective due to the water retention capacity, which is attracted by the covalent bonds present in the molecular structure and contributes directly to the increase in IEC. The smaller the thickness of the membrane, the smaller the internal resistance to the passage of water through it. Therefore, the reduction in thickness contributed to higher hydration [40], confirming the results presented in Fig. 4B.
The porosity of the BCH membranes was determined through the infiltration method according to the procedure described by Xu et al. [64]. Porosity is important, once in porous membranes there are fixed physical paths for passing through molecules. The porosity of the BCH studied varied between 73 and 91%, for thicknesses of 0.301 cm and 0.591 cm, respectively. As can be seen in Fig. 4C, there were changes in porosity for each of the thicknesses. There was a gradual increase as the thickness increased, which could be influenced by the overlapping of cellulose layers as a function of the time of the bioprocess [35]. In this case, the thickness of the membrane increases, forming a structure of suspended layers that are not cultured but more porous. Similar studies reported a porosity of ~ 94% for BCH, when synthesized under conditions like those presented in this research [34, 35].
Barros et al. [1] state that the time of biosynthesis directly influences the properties of bacterial cellulose, including porosity and mechanical resistance. The BCH porosity gives rise to two aligned pores of the bacterial cell and forms subfibrils ~ 1.5 nm in length that crystallize into microfibrils not cultured. The diameter of the microfibrils makes hydrated BCH much more porous. The presence of these pores and tunnels inside the hydrated film results in an intumescent fibrous structure, with water retention values of 100%, against 60% in vegetable cellulose [42].
It is possible to attribute the difference in the value of the porosity compared to other works, which are different for the physiological conditions of bacterial growth, such as the composition of the culture medium, pH, temperature, and oxygen concentration.
Tensile strength is an important mechanical characteristic of a PEM because low-resistance membranes can break during operation. The tensile strength and the elastic deformation coefficient were obtained from two tensile tests carried out on 4 samples of different thicknesses in triplicate. The experimental results obtained are presented in Table 2.
The results presented in Fig. 4D indicated that there was an increase, both in the maximum rupture stress and in the modulus of elasticity, with the increase in the thickness of BCH membranes. Membranes with 0.591 cm of thickness, present the best performance on mechanical properties, because this property depend to time of fermentation, a longer time, leads to a great polymerization degree increase and on the level of hydration too (Rebelo et al., 2018), therefore, mechanical strength increases [5]. It is important to remember that BCH is a biopolymer with an asymmetric structure, composed of regions with a high degree of crystalline ordering and others where the degree is low. These membranes contain various types of irregularities,therefore, the total surface area of a cellulose fiber is much greater than the surface area of an ideally smooth fiber of the same dimension, therefore, it shows mechanical anisotropic behavior it, with a high tensile modulus (0.058–2.481 MPa) along the fiber-layer direction but a low compressive modulus perpendicular to the stratified direction, some studies indicated this modulus is around 0.007 MPa [37].
The characterization of the specimens showed that the difference in thickness affects the way the membranes respond to stresses, which in turn support greater elongation. The sample with the greatest thickness (0.591 cm) showed a 30.86% greater strength compared to the sample with the smallest thickness (0.301 cm) and a 2.33% greater modulus of elasticity.
The thickest membrane had the highest modulus of elasticity. Thus, it is valid to add that the more compact the cellulose sheets are in the culture medium, there will be an increase in the crystallinity index and, consequently, in the thickness.
These values are linked to the hydrophilic nature and, therefore, to the thickness obtained by BCH during its formation, thus increasing the strength of the material by increasing its thickness.
The results of electrochemical impedance measurements on Fig. 5 indicate and increase on proton conductivity of BCH with the decrease of the thickness of the membrane. BCH Membrane with 0.301 cm and 0.591 cm of thickness showed a proton conductivity of 0.0114 and 0.0105 Scm−1, respectively, as soon as nafion membrane showed a proton conductivity of 0.137 Scm−1. In other tests it was found a proton conductivity of 0.0107 Scm−1 and 0.0106 Scm−1 for the membranes of 0.51 cm and 0.5552 cm, respectively. The smaller the thickness of the membrane, the smaller the internal resistance to the passage of protons through it. The data revealed that BCH (0.301 cm) also have highest water uptake and IEC than BCH (0.591 cm). Morin et al. [36] indicated that water content in membrane have positive effort to performance of the membrane so, hydration of membrane will create tunnel between clusters. The tunneling play key role on ionic conductivity of the membrane and help proton carrier, in the membrane [36].On the other hand, the nafion membrane presented higher proton conductivity than the BCH membranes, within its chemical structure it has sulfone groups that help, mainly, in the transport of protons, resulting in a value of 0.137 Scm−1.
The mass diffusion coefficient of oxygen (DO) is a measure of the oxygen permeability of membranes. In MFC, for example, it is important that the membranes do not allow oxygen crossover from the cathode into the anode chamber. Otherwise, the efficiency of the CCM in terms of chemical oxygen demand removal (COD) would be affected, affecting the growth of anaerobic microorganisms responsible for the oxidation of the substrate for the generation of electrons and finally causing the absence of current generation [41].
The DO value found in this research for a BCH membrane with a thickness of ~ 0.301 cm was 1.27 × 10−4 cm2 s−1 (Table 3), which is a value similar to clay membranes for MFC (1.3 × 10−4 cm2 s−1–9 × 10−5 cm2 s−1 [39] but much higher than those presented by Nafion (2.4 × 10−6 cm2 s−1) [27], and biopolymer PHA-PHB composite membranes (2.7 × 10−6 cm2 s−1) [41]. This can be attributed mainly to the membrane thickness and the presence of porous on BCH hydrogel membrane. In some studies, some substances as oly(glycidyl methacrylate) [10] and fucoidan [57] decrease CB porosity.
On the other hand, the mass diffusion coefficient of Ca2+ (DCa) obtained in the present work for a BCH membrane with a thickness of 0.301 cm was 1.084 × 10–6 cm2 s−1, slightly lower than that reported for Nafion membranes 8.4 × 10–6 cm2 s−1 [64]. Limitation of cation transfer due to decreased diffusion coefficients may contribute to a decline in the performance of MFCs. Likewise, a PEM with high DCa and low resistance must be developed to alleviate the scaling problems and, consequently, guarantee a long-term stable operation.
Conclusions
The best method of culture found was M5, where there was the addition of glycerol and allowed the production of homogeneous membranes and a higher kinetics reflected in the thickness of the membranes. In relation to the determined parameters, it was possible to observe that the IEC has a convex behavior in relation to the thickness of the BCH and the values of the pure BCH are close to zero. Likewise, it is suggested that, to improve ion transport, membranes should be functionalized or doped for applications such as PEM.
The water absorption capacity of BCH was greater than 70% for all thicknesses, prevailing the finer ones and proton conductivity showed the same tendence with thicnesses. The lower the thickness of the membrane, the lower the internal resistance to the passage of water through them. Therefore, the reduction of the thickness contributed to a greater hydration and more proton conductivity.
The mechanical resistance of the BCH membranes increased with their thickness. This allows, in applications such as PEM, a greater capacity for handling and operability.
The porosity has a gradual increase with the increase in thickness, which may be influenced by the overlapping of cellulose layers in function of the time of the bioprocess.
The mass diffusion coefficients of BCH membranes are high, but not as much as those reported by commercial membranes. Therefore, efforts must be invested to investigate different modifications in the membranes through the functionalization of BCH to improve characteristics such as IEC, porosity, and mass diffusivity.
References
Barros, M (2021) Propriedades de celulose bacteriana: influência do tempo de fermentação em diferentes cepas de Komagataeibacter. Master Dissertation, Universidade Federal do Ceará, Fortaleza, p 71
Budihardjo MA, Syafrudin, Effendi AJ et al (2021) Waste valorization using solid-phase microbial fuel cells (SMFCs): Recent trends and status. J Environ Manage 277:111417. https://doi.org/10.1016/j.jenvman.2020.111417
Caro-Astorga J, Lee K-Y, Ellis T (2022) Increasing bacterial cellulose compression resilience with glycerol or PEG400 for robuster engineered living materials. Carbohydr Polym Technol Appl 4:100245. https://doi.org/10.1016/j.carpta.2022.100245
Cazón P, Vázquez M (2021) Improving bacterial cellulose films by ex-situ and in-situ modifications: a review. Food Hydrocoll 113:106514. https://doi.org/10.1016/j.foodhyd.2020.106514
Chen S-Q, Lopez-Sanchez P, Wang D et al (2018) Mechanical properties of bacterial cellulose synthesised by diverse strains of the genus komagataeibacter. Food Hydrocoll 81:87–95. https://doi.org/10.1016/j.foodhyd.2018.02.031
Chen S-Q, Meldrum OW, Liao Q et al (2021) The influence of alkaline treatment on the mechanical and structural properties of bacterial cellulose. Carbohydr Polym 271:118431. https://doi.org/10.1016/j.carbpol.2021.118431
Chidanand NM, Eswara Prasad (2021) Renewable energy market by type (hydroelectric power, wind power, bioenergy, solar energy, and geothermal energy) and end use (residential, commercial, industrial, and others): global opportunity analysis and industry forecast, 2021–2030. In: https://www.alliedmarketresearch.com/renewable-energy-market
Costa AFS, Almeida FCG, Vinhas GM, Sarubbo LA (2017) Production of bacterial cellulose by Gluconacetobacter hansenii using corn steep liquor as nutrient sources. Front Microbiol. https://doi.org/10.3389/fmicb.2017.02027
Evans BR, O’Neill HM, Malyvanh VP et al (2003) Palladium-bacterial cellulose membranes for fuel cells. Biosens Bioelectron 18(7):917–923
Faria M, Vilela C, Mohammadkazemi F et al (2019) Poly(glycidyl methacrylate)/bacterial cellulose nanocomposites: preparation, characterization and post-modification. Int J Biol Macromol 127:618–627. https://doi.org/10.1016/j.ijbiomac.2019.01.133
Fortune Business Insights (2021) The proton exchange membrane fuel cell market is projected to grow from $2.10 billion in 2021 to $22.74 billion in 2028 at a CAGR of 40.6%. Read More at: https://www.fortunebusinessinsights.com/industry-reports/proton-exchange-membrane-fuel-cell-pemfc-market-101708
Gadim TDO, Figueiredo AGPR, Rosero-Navarro NC et al (2014) Nanostructured bacterial cellulose-poly(4-styrene sulfonic acid) composite membranes with high storage modulus and protonic conductivity. ACS Appl Mater Interfaces 6:7864–7875. https://doi.org/10.1021/am501191t
Gadim TDO, Loureiro FJA, Vilela C et al (2017) Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim Acta 233:52–61. https://doi.org/10.1016/j.electacta.2017.02.145
Ghozali M, Meliana Y, Chalid M (2021) Synthesis and characterization of bacterial cellulose by Acetobacter xylinum using liquid tapioca waste. Mater Today Proc 44:2131–2134. https://doi.org/10.1016/j.matpr.2020.12.274
Guerrero Moreno N, Cisneros Molina M, Gervasio D, Pérez Robles JF (2015) Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew Sust Energy Rev 52:897–906. https://doi.org/10.1016/j.rser.2015.07.157
Guilherme P, Borzone C, Bueno M, Lamour M (2016) Análise granulométrica de sedimentos arenosos de praias através de imagens digitais. Descrição de um protocolo de mensuração de partículas no software ImageJ - Fiji. Brazilian J Aquat Sci Technol. https://doi.org/10.14210/bjast.v19n2.6874
Ha JH, Shah N, Ul-Islam M et al (2011) Bacterial cellulose production from a single sugar α-linked glucuronic acid-based oligosaccharide. Process Biochem 46:1717–1723. https://doi.org/10.1016/j.procbio.2011.05.024
He Y, Wang D, Li Q et al (2020) Composite polymer electrolyte membranes based on nafion and modified PVDF electrospun nanofiber mats. J Wuhan Univ Technol-Mater Sci Ed 35:677–681. https://doi.org/10.1007/s11595-020-2306-5
Huang Y, Huang X, Ma M et al (2021) Recent advances on the bacterial cellulose-derived carbon aerogels. J Mater Chem C Mater 9:818–828. https://doi.org/10.1039/D0TC05433J
Huang Y, Xie A, Seidi F et al (2021) Core-shell heterostructured nanofibers consisting of Fe7S8 nanoparticles embedded into S-doped carbon nanoshells for superior electromagnetic wave absorption. Chem Eng J 423:130307. https://doi.org/10.1016/j.cej.2021.130307
Industry ARC (2021) Proton exchange membrane (PEM) fuel cells market – forecast (2022–2027) In: Industry ARC. https://www.industryarc.com/Report/15800/proton-exchange-membrane-pem-fuel-cells-market.html.. Accessed 30 Nov 2022
Jayashree S, Ramesh ST, Lavanya A et al (2019) Wastewater treatment by microbial fuel cell coupled with peroxicoagulation process. Clean Technol Environ Policy 21:2033–2045. https://doi.org/10.1007/s10098-019-01759-0
Jeon J-H, Oh I-K, Kee C-D, Kim S-J (2010) Bacterial cellulose actuator with electrically driven bending deformation in hydrated condition. Sens Actuators B Chem 146:307–313. https://doi.org/10.1016/j.snb.2010.02.046
Jeong CH, Ryu H, Zhang T et al (2018) Green tea powder supplementation enhances fermentation and antioxidant activity of set-type yogurt. Food Sci Biotechnol 27:1419–1427. https://doi.org/10.1007/s10068-018-0370-9
Jia Y-H, Ryu J-H, Kim CH et al (2012) Enhancing hydrogen production efficiency in microbial electrolysis cell with membrane electrode assembly cathode. J Ind Eng Chem 18:715–719
Jiang G, Qiao J, Hong F (2012) Application of phosphoric acid and phytic acid-doped bacterial cellulose as novel proton-conducting membranes to PEMFC. Int J Hydrog Energy 37:9182–9192. https://doi.org/10.1016/j.ijhydene.2012.02.195
Kim JR, Cheng S, Oh S-E et al (2007) Power generation using different cation, anion, and ultrafiltration membranes in microbial fuel cells. Environ Sci Technol 41:1004–1009. https://doi.org/10.1021/es062202m
Kotcharat P, Chuysinuan P, Thanyacharoen T et al (2021) Development of bacterial cellulose and polycaprolactone (PCL) based composite for medical material. Sustain Chem Pharm 20:100404. https://doi.org/10.1016/j.scp.2021.100404
Kuimov VM, Kryazhov AN, Yagupov AI et al (2022) Biopolymer-based membranes: green technologies for the separation of oil–water mixtures and the reduction of oil pollution. Clean Technol Environ Policy 24:1961–1985. https://doi.org/10.1007/s10098-022-02306-0
Laavanya D, Shirkole S, Balasubramanian P (2021) Current challenges, applications and future perspectives of SCOBY cellulose of kombucha fermentation. J Clean Prod 295:126454. https://doi.org/10.1016/j.jclepro.2021.126454
Lee K-Y, Quero F, Blaker JJ et al (2011) Surface only modification of bacterial cellulose nanofibres with organic acids. Cellulose 18:595–605. https://doi.org/10.1007/s10570-011-9525-z
Liu J, Wang S, Jiang L, Shao W (2021) Production and characterization of antimicrobial bacterial cellulose membranes with non-leaching activity. J Ind Eng Chem 103:232–238. https://doi.org/10.1016/j.jiec.2021.07.041
Malode SJ, Prabhu KK, Mascarenhas RJ et al (2021) Recent advances and viability in biofuel production. Energy Convers Manag: X. 10:100070. https://doi.org/10.1016/j.ecmx.2020.100070
Mautner A, Bismarck A (2021) Bacterial nanocellulose papers with high porosity for optimized permeance and rejection of nm-sized pollutants. Carbohydr Polym 251:117130. https://doi.org/10.1016/j.carbpol.2020.117130
Molina-Ramírez C, Castro M, Osorio Delgado M et al (2017) Effect of different carbon sources on bacterial nanocellulose production and structure using the low pH resistant strain komagataeibacter medellinensis. Materials 10:1. https://doi.org/10.3390/ma10060639
Morin A, Xu F, Gebel G, Diat O (2011) Influence of PEMFC gas flow configuration on performance and water distribution studied by SANS: Evidence of the effect of gravity. Int J Hydrog Energy 36:3096–3109. https://doi.org/10.1016/j.ijhydene.2010.11.070
Nakayama A, Kakugo A, Gong JP et al (2004) High mechanical strength double-network hydrogel with bacterial cellulose. Adv Funct Mater 14:1124–1128. https://doi.org/10.1002/adfm.200305197
Ndayisenga F, Yu Z, Kabera T et al (2021) Co-substrate facilitated charge transfer for bioelectricity evolution in a toxic blue-green alga-fed microbial fuel cell technology. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-021-02173-1
Neethu B, Bhowmick GD, Ghangrekar MM (2019) A novel proton exchange membrane developed from clay and activated carbon derived from coconut shell for application in microbial fuel cell. Biochem Eng J. 148:170–177. https://doi.org/10.1016/j.bej.2019.05.011
Ogungbemi E, Ijaodola O, Khatib FN et al (2019) Fuel cell membranes e Pros and cons. Energy 172:155–172. https://doi.org/10.1016/j.energy.2019.01.034
Olayiwola Sirajudeen AA, Mohamad Annuar MS, Ishak KA et al (2021) Innovative application of biopolymer composite as proton exchange membrane in microbial fuel cell utilizing real wastewater for electricity generation. J Clean Prod 278:123449. https://doi.org/10.1016/j.jclepro.2020.123449
Pacheco J, Marván E, Contreras M, Yee S (2004) Celulosa bacteriana en gluconacetobacter xylinum: biosíntesis y aplicaciones. Tip Revista Especializada en Ciencias Químico-Biológicas 7:18–25
Pardeshi PM, Mungray AA (2021) Performance of photopolymerized active layer forward osmosis membrane in the osmotic microbial fuel cell. Environ Technol Innov 23:101669. https://doi.org/10.1016/j.eti.2021.101669
Prathiba S, Kumar PS, Vo D-VN (2022) Recent advancements in microbial fuel cells: a review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere. 286:131856. https://doi.org/10.1016/j.chemosphere.2021.131856
Provin AP, Cubas ALV, de Dutra AR, A, Schulte NK, (2021) Textile industry and environment: can the use of bacterial cellulose in the manufacture of biotextiles contribute to the sector? Clean Technol Environ Policy 23:2813–2825. https://doi.org/10.1007/s10098-021-02191-z
Roger V, Fonty G, Andre C, Gouet P (1992) Effects of glycerol on the growth, adhesion, and cellulolytic activity of rumen cellulolytic bacteria and anaerobic fungi. Curr Microbiol 25:197–201. https://doi.org/10.1007/BF01570719
Santos BF, Ponezi AN, Fileti AMF (2016) Strategy for waste management in the production and application of biosurfactant through surface response methodology. Clean Technol Environ Policy 18:787–795. https://doi.org/10.1007/s10098-015-1052-4
Šegvić Klarić M, Kosalec I, Mastelić J et al (2007) Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett Appl Microbiol 44:36–42. https://doi.org/10.1111/j.1472-765X.2006.02032.x
Serra DO, Mika F, Richter AM, Hengge R (2016) The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and downregulating the biofilm regulator CsgD via the σE-dependent sRNA RybB. Mol Microbiol 101:136–151. https://doi.org/10.1111/MMI.13379
Shabani M, Younesi H, Pontié M et al (2021) Enhancement of microbial fuel cell efficiency by incorporation of graphene oxide and functionalized graphene oxide in sulfonated polyethersulfone membrane. Renew Energy 179:788–801. https://doi.org/10.1016/j.renene.2021.07.080
Sun B, Lin J, Wang T et al (2021) Gas assisted in situ biomimetic mineralization of bacterial cellulose/calcium carbonate bio composites by bacterial. Int J Biol Macromol 182:1690–1696. https://doi.org/10.1016/j.ijbiomac.2021.05.171
Szymańska M, Hoppe J, Dutkiewicz M et al (2022) Silicone polyether surfactant enhances bacterial cellulose synthesis and water holding capacity. Int J Biol Macromol. 208:642–653. https://doi.org/10.1016/j.ijbiomac.2022.03.124
Takata H, Nishikawa M, Arimura Y et al (2005) Study on water uptake of proton exchange membrane by using tritiated water sorption method. Int J Hydrog Energy 30:1017–1025. https://doi.org/10.1016/j.ijhydene.2005.02.006
Tomita Y, Kondo T (2009) Influential factors to enhance the moving rate of acetobacter xylinum due to its nanofiber secretion on oriented templates. Carbohydr Polym. 77:754–759. https://doi.org/10.1016/j.carbpol.2009.02.022
Tongphanpharn N, Guan C-Y, Chen W-S et al (2021) Evaluation of long-term performance of plant microbial fuel cells using agricultural plants under the controlled environment. Clean Technol Environ Policy. https://doi.org/10.1007/s10098-021-02222-9
Treviño-Garza MZ, Guerrero-Medina AS, González-Sánchez RA et al (2020) Production of microbial cellulose films from green tea (camellia sinensis) kombucha with various carbon sources. Coatings. https://doi.org/10.3390/coatings10111132
Vilela C, Silva ACQ, Domingues EM et al (2020) Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohyd Polym 230:115604. https://doi.org/10.1016/j.carbpol.2019.115604
Vilela C, Silvestre AJD, Figueiredo FML, Freire CSR (2019) Nanocellulose-based materials as components of polymer electrolyte fuel cells. J Mater Chem A Mater 7:20045–20074. https://doi.org/10.1039/C9TA07466J
Walkowiak-Kulikowska J, Wolska J, Koroniak H (2017) Polymers application in proton exchange membranes for fuel cells (PEMFCs). Phys Sci Rev. https://doi.org/10.1515/psr-2017-0018
Wang Y, Fang M, Zheng S et al (2021) Identification of Chinese green tea (Camellia sinensis) marker metabolites using GC/MS and UPLC-QTOF/MS. Food Sci Biotechnol 30:1293–1301. https://doi.org/10.1007/s10068-021-00970-4
Whiston M, Azevedo I, Shawn L et al (2019) Expert assessments of the cost and expected future performance of proton exchange membrane fuel cells for vehicles. Proc Natl Acad Sci 116:4899–4904. https://doi.org/10.1073/pnas.1804221116
Wu J-Y, Lay C-H, Chia SR et al (2021) Economic potential of bioremediation using immobilized microalgae-based microbial fuel cells. Clean Technol Environ Policy 23:2251–2264. https://doi.org/10.1007/s10098-021-02131-x
Wu Y, Huang T-Y, Li Z-X et al (2021) In-situ fermentation with gellan gum adding to produce bacterial cellulose from traditional Chinese medicinal herb residues hydrolysate. Carbohydr Polym 270:118350. https://doi.org/10.1016/j.carbpol.2021.118350
Xu J, Sheng GP, Luo HW et al (2012) Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res 46:1817–1824. https://doi.org/10.1016/j.watres.2011.12.060
Acknowledgements
The authors acknowledge the National Council for Scientific and Technological Development (CNPq) and the Research Center for Investigation and Development (CIDI) for financial support.
Funding
Open Access funding provided by Colombia Consortium. The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
MR-C: supervision, writing–reviewing and editing. MPG-G: methodology, data curation. LG-P: visualization, investigation. VP-R: data curation, investigation. TP-V: conceptualization, writing–original draft preparation, reviewing and editing. DH: funding acquisition, writing, reviewing and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ramírez-Carmona, M., Gálvez-Gómez, M.P., González-Perez, L. et al. Production of Bacterial Cellulose Hydrogel and its Evaluation as a Proton Exchange Membrane. J Polym Environ 31, 2462–2472 (2023). https://doi.org/10.1007/s10924-023-02759-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-023-02759-4