Abstract
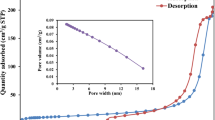
The present study employed the hydrothermal technique to graft biopolymer chitosan (CHS) with an aromatic aldehyde (salicylaldehyde, SA) for the adsorption of an acidic azo dye (reactive orange 16, RO 16) from an aqueous environment. The obtained hydrothermally achieved Schiff’s base (chitosan-salicylaldehyde, CHS-SA) material was analyzed using BET, CHN-O, FTIR, SEM, XRD, and pHpzc. The impacts of A: CHS-SA dosage (0.02–0.08 g/100 mL), B: pH (4–10), and C: time (5–25 min) factors on the adsorption performance of CHS-SA towards RO16 dye were fully examined using Box-Behnken design (BBD). The pseudo-second-order model exhibited the greatest match for the adsorption of RO16 dye by CHS-SA, whilst the equilibrium isotherm observations matched the Freundlich model. The maximal adsorption capacity of CHS-SA as established by the Langmuir model was 143.4 mg/g. Several pathways, including electrostatic attraction, π-π stacking, n-π interaction, and H-bonding drive the RO16 adsorption by CHS-SA. This work supports the idea of a hydrothermal way to produce grafted CHS-SA capable of removing harmful contaminants from an aquatic environment.








Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.”
References
Rathi BS, Kumar PS, Vo DVN (2021) Critical review on hazardous pollutants in water environment: occurrence, monitoring, fate, removal technologies and risk assessment. Sci Total Environ 797:149134
Abd Malek NN, Yousif E, Jawad EAH (2020) Optimization of adsorption parameters for reactive red 4 (RR4) removal by crosslinked chitosan-epichlorohydrin using Box Behnken design. Sci Lett 14(1):83–95
Deb A, Debnath A, Saha B (2021) Sono-assisted enhanced adsorption of eriochrome Black-T dye onto a novel polymeric nanocomposite: kinetic, isotherm, and response surface methodology optimization. J Disper Sci Technol 42(11):1579–1592
Seyedi MS, Sohrabi MR, Motiee F, Mortazavinik S (2020) Synthesis and characterization of activated carbon@ zerovalent iron–nickel nanoadsorbent for highly efficient removal of reactive orange 16 from aqueous sample: experimental design, kinetic, isotherm and thermodynamic studies. Res Chem Intermediate 46(3):1645–1662
Kumar D, Gupta SK (2022) Electrochemical oxidation of direct blue 86 dye using MMO coated Ti anode: modelling, kinetics and degradation pathway. Chem Eng Process-Process Intensifi 181:109127
Ihaddaden S, Aberkane D, Boukerroui A, Robert D (2022) Removal of methylene blue (basic dye) by coagulation-flocculation with biomaterials (bentonite and Opuntia ficus indica). J Water Process Eng 49:102952
Su M, Li H, He X, Xu Z (2022) Significant enhancement of pesticide and organic dyes degradation by ion-exchange within a metal–organic framework. Polyhedron 215:115651
Das P, Debnath P, Debnath A (2021) Enhanced sono-assisted adsorptive uptake of malachite green dye onto magnesium ferrite nanoparticles: kinetic, isotherm and cost analysis. Environ Nanotechnol Monit Manage 16:100506
Saha B, Debnath A, Saha B (2022) Fabrication of PANI@ Fe–Mn–Zr hybrid material and assessments in sono-assisted adsorption of methyl red dye: Uptake performance and response surface optimization. J Indian Chem Soc 99(9):100635
Rafaie HAG, Yusop NFM, Azmi NF, Abdullah NS, Ramli NIT (2021) Photocatalytic degradation of methylene blue dye solution using different amount of ZnO as a photocatalyst. Sci Lett 15(1):1–12
Jawad AH, Abdulhameed AS, Surip SN, Sabar S (2020) Adsorptive performance of carbon modified chitosan biopolymer for cationic dye removal: kinetic, isotherm, thermodynamic, and mechanism study. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1807966
Ma J, Faqir Y, Tan C, Khaliq G (2022) Terrestrial insects as a promising source of chitosan and recent developments in its application for various industries. Food Chem 373:131407
Mirzai M, Asadabadi S (2022) Magnetic nanocomposites containing low and medium-molecular weight chitosan for dye adsorption: hydrophilic property versus functional groups. J Polym Environ 30(4):1560–1573
Abd Malek NN, Jawad AH, Abdulhameed AS, Ismail K, Hameed BH (2020) New magnetic Schiff’s base-chitosan-glyoxal/fly ash/Fe3O4 biocomposite for the removal of anionic azo dye: an optimized process. Int J Biol Macromol 146:530–539
Rosli N, Yahya WZN, Wirzal MDH (2022) Crosslinked chitosan/poly (vinyl alcohol) nanofibers functionalized by ionic liquid for heavy metal ions removal. Int J Biol Macromol 195:132–141
Raval NP, Mukherjee S, Shah NK, Gikas P, Kumar M (2021) Hexametaphosphate cross-linked chitosan beads for the eco-efficient removal of organic dyes: Tackling water quality. J Environ Manage 280:111680
Priyadarshi G, Raval NP, Trivedi MH (2022) Microwave-assisted synthesis of cross-linked chitosan-metal oxide nanocomposite for methyl orange dye removal from unary and complex effluent matrices. Int J Biol Macromol 219:53–67
Chatterjee S, Chatterjee T, Woo SH (2011) Influence of the polyethyleneimine grafting on the adsorption capacity of chitosan beads for reactive black 5 from aqueous solutions. Chem Eng J 166(1):168–175
Rahmi R, Lelifajri L, Iqbal M, Fathurrahmi F, Jalaluddin J, Sembiring R, Iqhrammullah M (2022) Preparation, characterization and adsorption study of PEDGE-cross-linked magnetic chitosan (PEDGE-MCh) microspheres for Cd2+ removal. Arab J Sci Eng. https://doi.org/10.1007/s13369-022-06786-6
Khairkar SR, Pansare SV, Shedge AA, Chhatre S, Kulal DK, Patil VR, Pansare AV (2021) Biological macromolecule chitosan grafted co-polymeric composite: bio-adsorption probe on cationic dyes. Polym Bullet. https://doi.org/10.1007/s00289-021-03954-w
Chen J, Ouyang J, Chen W, Zheng Z, Yang Z, Liu Z, Zhou L (2022) Fabrication and adsorption mechanism of chitosan/Zr-MOF (UiO-66) composite foams for efficient removal of ketoprofen from aqueous solution. Chem Eng J 431:134045
Dodi G, Hritcu D, Lisa G, Popa MI (2012) Core–shell magnetic chitosan particles functionalized by grafting: synthesis and characterization. Chem Eng J 203:130–141
Tahira I, Aslam Z, Abbas A, Monim-ul-Mehboob M, Ali S, Asghar A (2019) Adsorptive removal of acidic dye onto grafted chitosan: a plausible grafting and adsorption mechanism. Int J Boil Macromol 136:1209–1218
Sanchez Ramirez DO, Periolatto M, Carletto RA, Varesano A, Vineis C, Tonetti C, Bongiovanni R (2020) Cr (VI) adsorption from aqueous solutions on grafted chitosan. Can J Chem Eng 98(7):1483–1494
Carvalho IC, Medeiros Borsagli FG, Mansur AA, Caldeira CL, Haas DJ, Lage AP, Mansur HS (2021) 3D sponges of chemically functionalized chitosan for potential environmental pollution remediation: biosorbents for anionic dye adsorption and ‘antibiotic-free’antibacterial activity. Environ Technol 42(13):2046–2066
Lu Y, Zhang W, Wang M, Zhang H, Li J, Luo W (2022) Fabrication of GO/PAN nanofiber membrane grafted with chitosan as efficient adsorbent for dye removal. J Polym Environ 30:2943–2954
Zhuang S, Zhang Q, Wang J (2021) Adsorption of Co2+ and Sr2+ from aqueous solution by chitosan grafted with EDTA. J Mol Liq 325:115197
Zia Q, Tabassum M, Meng J, Xin Z, Gong H, Li J (2021) Polydopamine-assisted grafting of chitosan on porous poly (L-lactic acid) electrospun membranes for adsorption of heavy metal ions. Int J Biol Macromol 167:1479–1490
Malesic-Eleftheriadou N, Evgenidou E, Lazaridou M, Bikiaris DN, Yang X, Kyzas GZ, Lambropoulou DA (2021) Simultaneous removal of anti-inflammatory pharmaceutical compounds from an aqueous mixture with adsorption onto chitosan zwitterionic derivative. Colloids Surf A Physicochem Eng Asp 619:126498
Chen Z, Zhang ZB, Zeng J, Zhang ZJ, Ma S, Tang CM, Xu JQ (2023) Preparation of polyethyleneimine-modified chitosan/Ce-UIO-66 composite hydrogel for the adsorption of methyl orange. Carbohydr Polym 299:120079
Vadivel T, Dhamodaran M, Kulathooran S, Kavitha S, Amirthaganesan K, Chandrasekaran S, Senguttuvan S (2020) Rhodium (III) complexes derived from complexation of metal with azomethine linkage of chitosan biopolymer Schiff base ligand: spectral, thermal, morphological and electrochemical studies. Carbohydr Res 487:107878
Sun Y, Kang Y, Zhong W, Liu Y, Dai Y (2020) A simple phosphorylation modification of hydrothermally cross-linked chitosan for selective and efficient removal of U (VI). J Solid State Chem 292:121731
Dalvand A, Nabizadeh R, Ganjali MR, Khoobi M, Nazmara S, Mahvi AH (2016) Modeling of reactive blue 19 azo dye removal from colored textile wastewater using L-arginine-functionalized Fe3O4 nanoparticles: optimization, reusability, kinetic and equilibrium studies. J Magne Magne Mater 404:179–189
Sing KS (1985) Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl Chem 57(4):603–619
El Kurdi R, Chebl M, Sillanpää M, El-Rassy H, Patra D (2021) Chitosan oligosaccharide/silica nanoparticles hybrid porous gel for mercury adsorption and detection. Mater Today Communicati 28:102707
Thatte CS, Rathnam MV, Pise AC (2014) Chitosan-based Schiff base-metal complexes (mn, Cu, Co) as heterogeneous, new catalysts for the β-isophorone oxidation. J Chem Sci 126(3):727–737
Dhanavel S, Manivannan N, Mathivanan N, Gupta VK, Narayanan V, Stephen A (2018) Preparation and characterization of cross-linked chitosan/palladium nanocomposites for catalytic and antibacterial activity. J Mol Liq 257:32–41
Jawad AH, Abdulhameed AS, Wilson LD, Hanafiah MAKM, Nawawi WI, ALOthman ZA, Rizwan Khan M (2021) Fabrication of schiff’s base chitosan-glutaraldehyde/activated charcoal composite for cationic dye removal: optimization using response surface methodology. J Polym Environ 29(9):2855–2868
de Araújo EL, Barbosa HFG, Dockal ER, Cavalheiro ÉTG (2017) Synthesis, characterization and biological activity of Cu (II), ni (II) and zn (II) complexes of biopolymeric Schiff bases of salicylaldehydes and chitosan. Int J Biol Macromol 95:168–176
Zhou S, Dong M, Ding X, Xue X, Yang H (2021) Application of RSM to optimize the recovery of ammonia nitrogen from high chromium effluent produced in vanadium industry using struvite precipitation. J Environ Chem Eng 9(6):106318
Kutluay S, Temel F (2021) Silica gel based new adsorbent having enhanced VOC dynamic adsorption/desorption performance. Colloids Surf A Physicochem Eng Asp 609:125848
Marichamy MK, Kumaraguru A, Jonna N (2021) Particle size distribution modeling and kinetic study for coagulation treatment of tannery industry wastewater at response surface optimized condition. J Clean Prod 297:126657
Obulapuram PK, Arfin T, Mohammad F, Khiste SK, Chavali M, Albalawi AN, Al-Lohedan HA (2021) Adsorption, equilibrium isotherm, and thermodynamic studies towards the removal of reactive orange 16 dye using Cu (I)-polyaninile composite. Polym 13(20):3490
Jawad AH, Abdulhameed AS, Hanafiah MAKM, ALOthman ZA, Khan MR, Surip SN (2021) Numerical desirability function for adsorption of methylene blue dye by sulfonated pomegranate peel biochar: modeling, kinetic, isotherm, thermodynamic, and mechanism study. Korean J Chem Eng 38(7):1499–1509
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Vet Akad Handl 24:1–39
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. Chem Eng J 70:115–124
Ahmed MA, Ahmed MA, Mohamed AA (2022) Facile adsorptive removal of dyes and heavy metals from wastewaters using magnetic nanocomposite of Zinc ferrite@ reduced graphene oxide. Inorg Chem Communi 144:109912
Abdulhameed AS, Mohammad AT, Jawad AH (2019) Modeling and mechanism of reactive orange 16 dye adsorption by chitosan-glyoxal/TiO2 nanocomposite: application of response surface methodology. Desalin Water Treat 164:346–360
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Frenudlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Temkin MI (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Wang W, Fan M, Ni J, Peng W, Cao Y, Li H, Song S (2022) Efficient dye removal using fixed-bed process based on porous montmorillonite nanosheet/poly (acrylamide-co-acrylic acid)/sodium alginate hydrogel beads. Appl Clay Sci 219:106443
Kloster GA, Valiente M, Marcovich NE, Mosiewicki MA (2020) Adsorption of arsenic onto films based on chitosan and chitosan/nano-iron oxide. Int J Biol Macromol 165:1286–1295
Arfin T, Bhaisare DA, Waghmare SS (2021) Development of a PANI/Fe (NO3)2 nanomaterial for reactive orange 16 (RO16) dye removal. Anal Methods 13(44):5309–5327
Abd Malek NN, Jawad AH, Ismail K, Razuan R, ALOthman ZA (2021) Fly ash modified magnetic chitosan-polyvinyl alcohol blend for reactive orange 16 dye removal: Adsorption parametric optimization. Int J Boil Macromol 189:464–476
Piri F, Mollahosseini A, Hosseini MM (2019) Enhanced adsorption of dyes on microwave-assisted synthesized magnetic zeolite-hydroxyapatite nanocomposite. J Environ Chem Eng 7(5):103338
Akbar Ali AM, Karthikeyan RK, Sentamil Selvan M, Mithilesh KR, Madhangi P, Maheswari N, Janani SG, Padmanaban VC, Singh RS (2020) Removal of reactive orange 16 by adsorption onto activated carbon prepared from rice husk ash: statistical modelling and adsorption kinetics. Sep Sci Technol 55(1):26–34
Acknowledgements
The authors gratefully acknowledge the Ministry of Higher Education Malaysia (MoHE) for supporting this research project under Fundamental Research Grant Scheme (Ref: FRGS/1/2022/TK09/UITM/02/26).
Funding
This research project has received a Fundamental Research Grant Scheme (Ref: FRGS/1/2022/TK09/UITM/02/26) fund from the Ministry of Higher Education (MoHE) Malaysia.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by NIN, ASA, AHJ, SNS, RR, MLI. The first draft of the manuscript was written by NIN, ASA, AHJ, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Normi, N.I., Abdulhameed, A.S., Jawad, A.H. et al. Hydrothermal-Assisted Grafting of Schiff base Chitosan by Salicylaldehyde for Adsorptive Removal of Acidic Dye: Statistical Modeling and Adsorption Mechanism. J Polym Environ 31, 1925–1937 (2023). https://doi.org/10.1007/s10924-022-02730-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02730-9