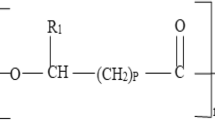
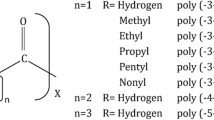
Abstract
In recent decades, the global accumulation of plastics and the resulting pollution, as well as the increase in the price of oil, have driven studies aimed at the production of biodegradable plastics and the use of renewable carbon sources for their synthesis. In this context, the poly(hydroxyalkanoate) (PHA) biopolymers are highlighted as a class type of microbial polyester produced by fermentation of carbon substrates and stored in the form of granules in the bacterial cytoplasm. The scientific-industrial prominence of PHAs is due to their characteristics, such as thermoplasticity, biodegradable, non-toxicity, and biocompatibility. This versatility makes these biopolymers potential candidates for industrial, pharmaceutical, biomedical and food packaging applications. Despite these advantages, the commercialization of PHA on a large scale is restricted by its high production cost compared to petroleum-based plastics. The high cost is mainly attributed to the carbon source used in the fermentation processes, representing approximately 50% of the total production costs. Thereby, studies have focused on prospecting alternative carbon sources, such as agro-industrial residues to reduce the production cost to make PHAs more commercially competitive. In this regard, this review presents an overview of poly(hydroxyalkanoates), addressing alternative carbon sources as substrates for their synthesis, description of analytical and extraction methods applied to recover and characterize this biopolymer.
Similar content being viewed by others
References
Gan Z, Zhang H (2019) PMBD: A comprehensive plastics microbial biodegradation database. Database. https://doi.org/10.1093/database/baz119
Towler G, Sinnott R (2021) Chemical engineering design: Materials of construction Butterworth-Heinemann. Elsevier, Amsterdam
Zimmermann L, Dierkes G, Ternes TA, Völker C, Wagner M (2019) Benchmarking the in vitro toxicity and chemical composition of plastic consumer products. Environ Sci Technol 53:11467–11477. https://doi.org/10.1021/acs.est.9b02293
Roosen M, Mys N, Kusenberg M, Billen P, Dumoulin A, Dewulf J, Van Geem KM, Ragaert K, De Meester S (2020) Detailed analysis of the composition of selected plastic packaging waste products and its implications for mechanical and thermochemical recycling. Environ Sci Technol 54:13282–13293. https://doi.org/10.1021/acs.est.0c03371
Levidow L, Raman S (2019) Metamorphosing waste as a resource: Scaling waste management by ecomodernist means. Geoforum 98:108–122. https://doi.org/10.1016/j.geoforum.2018.10.020
Okoffo ED, Donner E, McGrath SP, Tscharke BJ, O’Brien JW, O’Brien S, Ribeiro F, Burrows SD, Toapanta T, Rauert C, Samanipour S, Mueller JF, Thomas KV (2021) Plastics in biosolids from 1950 to 2016: A function of global plastic production and consumption. Water Res 201:117367. https://doi.org/10.1016/j.watres.2021.117367
Soares J, Miguel I, Venâncio C, Lopes I, Oliveira M (2021) Public views on plastic pollution: Knowledge, perceived impacts, and pro-environmental behaviours. J Hazard Mater 412:125227. https://doi.org/10.1016/j.jhazmat.2021.125227
Moharir RV, Kuma S (2019) Challenges associated with plastic waste disposal and allied microbial routes for its effective degradation: A comprehensive review. J Clean Prod 208:65–76. https://doi.org/10.1016/j.jclepro.2018.10.059
Ranjbari M, Esfandabadi ZS, Shevchenko T, Chassagnon-Haned N, Peng W, Tabatabaei M, Aghbashlo M, l, (2022) Mapping healthcare waste management research: Past evolution, current challenges, and future perspectives towards a circular economy transition. J Hazar Mater 422:126724. https://doi.org/10.1016/j.jhazmat.2021.126724
Geyer R, Jambeck JR, Law KL (2017) Production, use, and fate of all plastics ever made. Sci Adv. https://doi.org/10.1126/sciadv.1700782
Lebreton L, Andrady A (2019) Future scenarios of global plastic waste generation and disposal. Palgrave Commun. https://doi.org/10.1057/s41599-018-0212-7
Chamas A, Moon H, Zheng J, Qiu Y, Tabassum T, Jang JH, Abu-Omar M, Scott SL, Suh S (2020) Degradation rates of plastics in the environment. ACS Sustain Chem Eng 8:3494–3511. https://doi.org/10.1021/acssuschemeng.9b06635
Browning S, Beymer-Farris B, Seay JR (2021) Addressing the challenges associated with plastic waste disposal and management in developing countries. Curr Opin Chem Eng 32:100682. https://doi.org/10.1016/j.coche.2021.100682
Tiseo I (2021) Plastic production forecast worldwide 2025–2050. https://www.statista.com/statistics/664906/plastics-production-volume-forecast-worldwide. Accessed 18th Aug 2021.
Thiruchelvi R, Das A, Sikdar E (2021) Bioplastics as better alternative to petro plastic. Mater Today: Proc 37:1634–1639. https://doi.org/10.1016/j.matpr.2020.07.176
Havstad M R (2020) In: Letcher TM (ed) Plastic Waste and Recycling. Academic Press, Cambridge.
Yin GZ, Yang XM (2020) Biodegradable polymers: a cure for the planet, but a long way to go. J Polym Res 27:38. https://doi.org/10.1007/s10965-020-2004-1
Jain A, Sarsaiya S, Awasthi MK, Singh R, Rajput R, Mishra UC, Chen J, Shia J (2022) Bioenergy and bio-products from bio-waste and its associated modern circular economy: Current research trends, challenges, and future outlooks. Fuel 307:121859. https://doi.org/10.1016/j.fuel.2021.121859
Ortelli S, Costa AL, Torri C, Samorì C, Galletti P, Vineis C, Varesano A, Bonura L, Bianchi G (2019) Innovative and sustainable production of biopolymers. In: Tolio T, Copani G, Terkaj W (eds) Factories of the Future. Springer, Cham
Verma ML, Kumar K, Jeslin J, Dubey NK (2020) Microbial production of biopolymers with potential biotechnological applications. In: Dean M (ed) Biopolymer-Based Formulations. Elsevier, Amsterdam
Vinod A, Sanjay MR, Suchart S, Jyotishkumar P (2020) Renewable and sustainable biobased materials: An assessment on biofibers, biofilms, biopolymers and biocomposites. J Clean Prod 258:120978. https://doi.org/10.1016/j.jclepro.2020.120978
Nasrollahzadeh M, Sajjadi M (2021) Synthesis of biopolymer-based metal nanoparticles. In: Dennis S (ed) Biopolymer-Based Metal Nanoparticle Chemistry for Sustainable Applications. Elsevier, Amsterdam
George A, Sanjay MR, Srisuk R, Parameswaranpillai J, Siengchin S (2020) A comprehensive review on chemical properties and applications of biopolymers and their composites. Int J Biol Macromol 154:329–338. https://doi.org/10.1016/j.ijbiomac.2020.03.120
Moradali MF, Rehm BHA (2020) Bacterial biopolymers: from pathogenesis to advanced materials. Nat Rev Microbiol 18:195–210. https://doi.org/10.1038/s41579-019-0313-3
Bugnicourt E, Cinelli P, Lazzeri A, Alvarez VA (2014) Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym Lett 8:791–808
Confente I, Scarpi D, Russo I (2020) Marketing a new generation of bio-plastics products for a circular economy: The role of green self-identity, self-congruity, and perceived value. J Bus Res 112:431–439. https://doi.org/10.1016/j.jbusres.2019.10.030
Fernández-DaCosta C, Posada JA, Kleerebezem R, Cuellar MC, Ramirez A (2015) Microbial community-based polyhydroxyalkanoates (PHAs) production from wastewater: techno-economic analysis and ex-ante environmental assessment. Bioresour Technol 185:368–377. https://doi.org/10.1016/j.biortech.2015.03.025
Choi SY, Rhie MN, Kim HT, Cho JJC, IJ, Son J, Jo SY, Sohn YJ, Baritugo KA, Pyo J, Lee Y, Lee SY, Park SJ, (2019) Metabolic engineering for the synthesis of polyesters: A 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters. Metab Eng 58:47–48. https://doi.org/10.1016/j.ymben.2019.05.009
Sabbagh F, Muhamad II (2017) Production of poly-hydroxyalkanoate as secondary metabolite with main focus on sustainable energy. Renew Sustain Energy Rev 72:95–104. https://doi.org/10.1016/j.rser.2016.11.012
Sagong HY, Son HF, Choi SY, Lee SY, Kim KJ (2018) Structural insights into polyhydroxyalkanoates biosynthesis. Trends Biochem Sci 43:790–805. https://doi.org/10.1016/j.tibs.2018.08.005
Kovalcik A, Sangroniz L, Kalina M, Skopalova K, Humpolíček P, Omastova M, Mundigler N, Müller AJ (2020) Properties of scaffolds prepared by fused deposition modeling of poly(hydroxyalkanoates). Int J Biol Macromol 161:364–376. https://doi.org/10.1016/j.ijbiomac.2020.06.022
Cabrera F, Torres A, Campos JL, Jeison D (2019) Effect of operational conditions on the behaviour and associated costs of mixed microbial cultures for PHA production. Polymers 11:191. https://doi.org/10.3390/polym11020191
Kato N (2019) Production of crude bioplastic-beads with microalgae: Proof-of-concept. Bioresour Technol Rep 6:81–84. https://doi.org/10.1016/j.biteb.2019.01.022
García-Quiles L, Fernández Cuello A, Castell P (2019) Sustainable materials with enhanced mechanical properties based on industrial polyhydroxyalkanoates reinforced with organomodified sepiolite and montmorillonite. Polymers 11(4):696. https://doi.org/10.3390/polym11040696
Zheng Y, Chen JC, Ma YM, Chen GQ (2020) Engineering biosynthesis of polyhydroxyalkanoates (PHA) for diversity and cost reduction. Metab Eng 58:82–93. https://doi.org/10.1016/j.ymben.2019.07.004
De Donno NL, Moreno Sayavedra S, Rene ER (2021) Polyhydroxyalkanoate (PHA) production via resource recovery from industrial waste streams: A review of techniques and perspectives. Bioresour Technol 331:124985. https://doi.org/10.1016/j.biortech.2021.124985
Koller M (2018) Biodegradable and biocompatible polyhydroxy-alkanoates (PHA): Auspicious microbial macromolecules for pharmaceutical and therapeutic applications. Molecules 23:362. https://doi.org/10.3390/molecules23020362
Koller M (2019) Polyhydroxyalkanoate biosynthesis at the edge of water activitiy-haloarchaea as biopolyester factories. Bioengineering 6:34. https://doi.org/10.3390/bioengineering6020034
Alcântara JMG, Distante F, Storti G, Moscatelli D, Morbidelli M, Sponchioni M (2020) Current trends in the production of biodegradable bioplastics: The case of polyhydroxyalkanoates. Biotechnol Adv 42:107582. https://doi.org/10.1016/j.biotechadv.2020.107582
Bhattacharyya B, Behera HT, Mojumdar A, Raina V, Ray L (2019) Polyhydroxyalkanoates: resources, demands and sustainability. In: Jamil N, Kumar P, Batool R (eds) Soil Microenvironment for Bioremediation and Polymer Production, 1st edn. Wiley, Hoboken
Gadgil BST, Killi N, Rathna GVN (2017) Polyhydroxyalkanoates as biomaterials Med Chem Comm 8:1774–1787. https://doi.org/10.1039/C7MD00252A
Li Z, Loh XJ (2015) Water soluble polyhydroxyalkanoates: future materials for therapeutic applications. Chem Soc Rev 44:2865–2879. https://doi.org/10.1039/C5CS00089K
Li Z, Yang J, Loh X (2016) Polyhydroxyalkanoates: opening doors for a sustainable future. NPG Asia Mater. https://doi.org/10.1038/am.2016.48
Chek MF, Kim SY, Mori T, Arsad H, Samian MR, Sudesh K, Hakoshima T (2017) Structure of polyhydroxyalkanoate (PHA) synthase PhaC from Chromobacterium sp. USM2, producing biodegradable plastics. Sci Rep 7:5312. https://doi.org/10.1038/s41598-017-05509-4
Feghali E, Tauk L, Ortiz P, Vanbroekhoven K, Eevers W (2020) Catalytic chemical recycling of biodegradable polyesters. Polym Degrad Stab 179:109241. https://doi.org/10.1016/j.polymdegradstab.2020.109241
Kootstra AMJ, Elissen HJH, Huurman S (2017) PHA’s (Polyhydroxyalkanoates): General information on structure and raw materials for their production. Wageningen, UR. https://edepot.wur.nl/414011.
Khunthongkaew P, Murugan P, Sudesh K, Iewkittayakorn J (2018) Biosynthesis of polyhydroxyalkanoates using Cupriavidus necator H16 and its application for particleboard production. J Polym Res 25:131. https://doi.org/10.1007/s10965-018-1521-7
Rai R, Keshavarz T, Roether JA, Boccaccini AR, Roy I (2011) Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng R Rep 72:29–47. https://doi.org/10.1016/j.mser.2010.11.002
Raza ZA, Riaz S, Banat IM (2018) Polyhydroxyalkanoates: properties and chemical modification approaches for their functionalization. Biotechnol Prog 34:29–41. https://doi.org/10.1002/btpr.2565
Obruca S, Sedlacek P, Koller M, Kucera D, Pernicova I (2018) Involvement of polyhydroxyalkanoates in stress resistance of microbial cells: Biotechnological consequences and applications. Biotechnol Adv 36:856–870. https://doi.org/10.1016/j.biotechadv.2017.12.006
Zou H, Shi M, Zhang T, Li L, Li L, Xian M (2017) Natural and engineered polyhydroxyalkanoate (PHA) synthase: key enzyme in biopolyester production. Appl Microbiol Biotechnol 101:7417–7426. https://doi.org/10.1007/s00253-017-8485-0
Zinn M (2003) Tailor-made synthesis of polyhydroxyalkanoate. Eur cells mater 5:38–39
Dwivedi R, Pandey R, Kumar S, Mehrotra D (2020) Polyhydroxyalkanoates (PHA): role in bone scaffolds. J Oral Biol Craniofac Res 10:389–392. https://doi.org/10.1016/j.jobcr.2019.10.004
Sim SJ, Snell KD, Hogan SA, Stubbe J, Rha C, Sinskey AJ (1997) PHA synthase activity controls the molecular weight and polydispersity of polyhydroxybutyrate in vivo. Nat Biotechnol 15:63–67. https://doi.org/10.1038/nbt0197-63
Matsumoto K, Kageyama Y (2019) Increased production and molecular weight of artificial polyhydroxyalkanoate poly (2-hydroxybutyrate) above the glass transition temperature threshold. Front Bioeng Biotechnol 7:177. https://doi.org/10.3389/fbioe.2019.00177
Cruz MV, Araújo D, Alves VD, Freitas F, Reis MA (2016) Characterization of medium chain length polyhydroxyalkanoate produced from olive oil deodorizer distillate. Int J Biol Macromol 82:243–248. https://doi.org/10.1016/j.ijbiomac.2015.10.043
Winnacker M (2019) Polyhydroxyalkanoates: Recent advances in their synthesis and applications. Eur J Lipid Sci Technol 121:1900101. https://doi.org/10.1002/ejlt.201900101
Cruz RAP, Oehmen A, Reis MAM (2022) The impact of biomass withdrawal strategy on the biomass selection and polyhydroxyalkanoates accumulation of mixed microbial cultures. New Biotechnol 66:8–15. https://doi.org/10.1016/j.nbt.2021.08.004
Muhammadi S, Afzal M, Hameed S (2015) Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: production, biocompatibility, biodegradation, physical properties and applications. Green Chem Lett Rev 8:56–77. https://doi.org/10.1080/17518253.2015.1109715
Koller M, Maršálek L, Dias MMS, Braunegg G (2017) Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol 37:24–38. https://doi.org/10.1016/j.nbt.2016.05.001
Jendrossek D (2009) Polyhydroxyalkanoate granules are complex subcellular organelles (carbonosomes). J Bacteriol 191:3195–3202. https://doi.org/10.1128/JB.01723-08
Mozejko-ciesielska J, Szacherska K, Marciniak P (2019) Pseudomonas species as producers of eco-friendly polyhydroxyalkanoates. J Polym Environ 27:1151–1166. https://doi.org/10.1016/j.jclepro.2018.10.059
Slaninova E, Sedlacek P, Mravec F, Mullerova L, Samek O, Koller M, Hesko O, Kucera D, Marova I, Obruca S (2018) Light scattering on PHA granules protects bacterial cells against the harmful effects of UV radiation. Appl Microbiol Biotechnol 102:1923–1931. https://doi.org/10.1007/s00253-018-8760-8
Sedlacek P, Slaninova E, Koller M, Nebesarova J, Marova I, Krzyzanek V, Obruca S (2019) PHA granules help bacterial cells to preserve cell integrity when exposed to sudden osmotic imbalances. New Biotechnol 49:129–136. https://doi.org/10.1016/j.nbt.2018.10.005
Obruca S, Sedlacek P, Slaninova E, Fritz I, Daffert C, Meixner K, Sedrlova Z, Koller M (2020) Novel unexpected functions of PHA granules. Appl Microbiol Biotechnol 104:4795–4810. https://doi.org/10.1007/s00253-020-10568-1
Tang R, Weng C, Peng X, Han Y (2020) Metabolic engineering of Cupriavidus necator H16 for improved chemoautotrophic growth and PHB production under oxygen-limiting conditions. Metab Eng 61:11–23. https://doi.org/10.1016/j.ymben.2020.04.009
Meng DC, Shen R, Yao H, Chen JC, Wu Q, Chen GQ (2014) Engineering the diversity of polyesters. Curr Opin Biotechnol 29:24–33. https://doi.org/10.1016/j.copbio.2014.02.013
Thomas T, Elain A, Bazire A, Bruzaud S (2019) Complete genome sequence of the halophilic PHA-producing bacterium Halomonas sp. SF2003: insights into its biotechnological potential. World J Microbiol Biotechnol 35:50. https://doi.org/10.1007/s11274-019-2627-8
Chek MF, Kim SY, Mori T, Tan HT, Sudesh K, Hakoshima T (2020) Asymmetric open-closed dimer mechanism of polyhydroxyalkanoate synthase PhaC. Iscience 23:101084. https://doi.org/10.1016/j.isci.2020.101084
Zhang W, Chen C, Cao R, Maurmann L, Li P (2015) Inhibitors of polyhydroxyalkanoate (PHA) synthases: Synthesis, molecular docking, and implications. ChemBioChem 16:156–166. https://doi.org/10.1002/cbic.201402380
Mezzolla V, D’urso OF, Poltronieri P, (2018) Role of PhaC type I and Type II enzymes during PHA biosynthesis. Polymers 10(8):910. https://doi.org/10.3390/polym10080910
Parlane NA, Gupta SK, Rubio-Reyes P, Chen S, Gonzalez-Miro M, Wedlock DN, Rehm BH (2016) Self-assembled protein-coated polyhydroxyalkanoate beads: properties and biomedical applications. ACS Biomater Sci Eng 3:3043–3057. https://doi.org/10.1021/acsbiomaterials.6b00355
Mukheem A, Shahabuddin S, Akbar N, Anwar A, Sarih NM, Sudesh K, Khan NA, Sridewi N (2020) Fabrication of biopolymer polyhydroxyalkanoate/chitosan and 2Dmolybdenum disulfide-doped scaffolds for antibacterial and biomedical applications. Appl Microbiol Biotechnol 104:3121–3131. https://doi.org/10.1007/s00253-020-10416-2
Luo Z, Wu YL, Li Z, Loh XJ (2019) Recent progress in polyhydroxyalkanoates-based copolymers for biomedical applications. Biotechnol J 14:1900283. https://doi.org/10.1002/biot.201900283
Zhang J, Shishatskaya EI, Volova TG, Da Silva LF, Chen GQ (2018) Polyhydroxyalkanoates (PHA) for therapeutic applications. Mater Sci Eng C 86:144–150. https://doi.org/10.1016/j.msec.2017.12.035
Keskin G, Kizil G, Bechelany M, Pochat-Bohatier C, Öner M (2017) Potential of polyhydroxyalkanoate (PHA) polymers family as substitutes of petroleum based polymers for packaging applications and solutions brought by their composites to form barrier materials. Pure Appl Chem 89:1841–1848. https://doi.org/10.1515/pac-2017-0401
Yadav B, Talan A, Tyagi RD, Drogui P (2021) Concomitant production of value-added products with polyhydroxyalkanoate (PHA) synthesis: A review. Bioresour Technol 337:125419. https://doi.org/10.1016/j.biortech.2021.125419
Akaraonye E, Keshavarz T, Roy I (2010) Production of polyhydroxyalkanoates: The future green materials of choice. J Chem Technol Biotechnol 85:732–743. https://doi.org/10.1002/jctb.2392
Pérez-Arauz AO, Aguilar-Rabiela AE, Vargas-Torres A, Rodríguez-Hernández AI, Chavarría-Hernández N, Vergara-Porras B, López-Cuellar MR (2019) Production and characterization of biodegradable films of a novel polyhydroxyalkanoate (PHA) synthesized from peanut oil. Food Packag Shelf Life 20:100297. https://doi.org/10.1016/j.fpsl.2019.01.001
Singh AK, Sharma L, Srivastava JK, Mallick N, Ansari MI (2018) Microbially Originated Polyhydroxyalkanoate (PHA) Biopolymers: An Insight into the Molecular Mechanism and Biogenesis of PHA Granules. In: Singh O, Chandel A (eds) Sustainable Biotechnology-Enzymatic Resources of Renewable Energy. Springer, Cham
Ray S, Kalia VC (2017) Biomedical applications of polyhydroxyalkanoates. Indian J Microbiol 57:261–269. https://doi.org/10.1007/s12088-017-0651-7
Yao CL, Chen JH, Lee CH (2018) Effects of various monomers and micro-structure of polyhydroxyalkanoates on the behavior of endothelial progenitor cells and endothelial cells for vascular tissue engineering. J Polym Res 25:187. https://doi.org/10.1007/s10965-017-1341-1
Kalia VC, Ray S, Patel SK, Singh M, Singh GP (2019) The Dawn of Novel Biotechnological Applications of Polyhydroxyalkanoates. In: Kalia V (ed) Biotechnological Applications of Polyhydroxyalkanoates. Springer, Singapore
Sabarinathan D, Chandrika SP, Venkatraman P, Easwaran M, Sureka CS, Preethia K (2018) Production of polyhydroxybutyrate (PHB) from Pseudomonas plecoglossicida and its application towards cancer detection. Inform Med Unlocked 11:61–67. https://doi.org/10.1016/j.imu.2018.04.009
Chen GQ (2010) Introduction of Bacterial Plastics PHA, PLA, PBS, PE, PTT, and PPP. In: Chen GGQ (ed) Plastics from bacteria. Springer, Berlin
Shershneva A, Murueva A, Nikolaeva E, Shishatskaya E, Volova T (2017) Novel spray-dried PHA microparticles for antitumor drug release. Dry Technol 36:1387–1398. https://doi.org/10.1080/07373937.2017.1407940
Lam W, Wang Y, Chan PL, Chan SW, Tsang YF, Chua H, Yu PHF (2017) Production of polyhydroxyalkanoates (PHA) using sludge from different wastewater treatment processes and the potential for medical and pharmaceutical applications. Environ Technol 38:1779–1791. https://doi.org/10.1080/09593330.2017.1316316
McColgan-Bannon KI, Upson S, Gentile P, Tausif M, Russell S, Dalgarno K, Ferreira AM (2019) Biomimetic properties of force-spun PHBV membranes functionalised with collagen as substrates for biomedical application. Coatings 9:350. https://doi.org/10.3390/coatings9060350
Cinelli P, Mallegni N, Gigante V, Montanari A, Seggiani M, Coltelli MB, Lazzeri A (2019) Biocomposites based on polyhydroxyalkanoates and natural fibres from renewable byproducts. Appl Food Biotechnol 6:35–43
Keunun P, Rakkarn T, Yunu T, Paichid N, Prasertsan P, Sangkharak K (2018) The production of polyhydroxybutyrate by two-step fermentation and the application of polyhydroxybutyrate as a novel substrate for a biolubricant. J Polym Environ 26:2459–2466. https://doi.org/10.1007/s10924-017-1140-0
Xu P, Yang W, Niu D, Yu M, Du M, Dong W, Chen M, Lemstra PJ, Ma P (2020) Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem Eng Sci 382:122864. https://doi.org/10.1016/j.cej.2019.122864
Sangkharak K (2020) Novel polyhydroxyalkanoate-based biocomposites obtained by solution casting and their application for bacteria removal and domestic wastewater purification. J Polym Environ 28:1893–1900. https://doi.org/10.1007/s10924-020-01738-3
Li W (2020) Bacteria-triggered release of a potent biocide from core-shell polyhydroxyalkanoate (PHA)-based nanofibers for wound dressing applications. J Biomater Sci Polym Ed 31:394–406. https://doi.org/10.1080/09205063.2019.1693882
Jiang L, Luo Z, Loh XJ, Wu YL, Li Z (2019) PHA-based thermogel as a controlled zero-order chemotherapeutic delivery system for the effective treatment of melanoma. ACS ACS Appl Bio Mater 2:3591–3600. https://doi.org/10.1021/acsabm.9b00467
Gao X, Chen JC, Wu Q, Chen GQ (2011) Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr Opin Biotechnol 22:768–774. https://doi.org/10.1016/j.copbio.2011.06.005
Choudhury AKR (2018) Biopolymers in textile industry. Apple Academic Press, Toronto, Biopolymers and biomaterials
Amelia TSM, Govindasamy S, Tamothran AM, Vigneswari S, Bhubalan K (2019) Applications of PHA in agriculture. In: Kalia VC (ed) Biotechnological Applications of Polyhydroxyalkanoates, 1st edn. Springer Nature, Singapore
Chandravadee R, Sangkharak K, Pechsiri J (2020) Application of polyhydroxyalkanoates as carbon source for nitrogen compound treatment in recirculating aquaculture system (RAS). Thaksin J 23:1–10
Jain R, Tiwari A (2015) Biosynthesis of planet friendly bioplastics using renewable carbon source. J Environ Health Sci Engineer 13:11. https://doi.org/10.1186/s40201-015-0165-3
Rahman MH, Bhoi PR (2021) An overview of non-biodegradable bioplastics. J Clean Prod 294:126218. https://doi.org/10.1016/j.jclepro.2021.126218
Narancic T, Cerrone F, Beagan N, O’Connor KE (2020) Recent advances in bioplastics: Application and biodegradation. Polymers 12:920. https://doi.org/10.3390/polym12040920
Mehrpouya M, Vahabi H, Barletta M, Laheurte P, Langloise V (2021) Additive manufacturing of polyhydroxyalkanoates (PHAs) biopolymers: Materials, printing techniques, and applications. Mater Sci Eng C 127:112216. https://doi.org/10.1016/j.msec.2021.112216
Volova TG, Boyandin AN, Vasiliev AD, Karpov VA, Prudnikova SB, Mishukova OV, Boyarskikh UA, Filipenko ML, Rudnev VP, Xuân BB, Dũng VV, Gitelson II (2010) Biodegradation of polyhydroxyalkanoates (PHAs) in tropical coastal waters and identification of PHA-degrading bacteria. Polym Degrad Stab 95:2350–2359. https://doi.org/10.1016/j.polymdegradstab.2010.08.023
Urbanek AK, Mirończuk AM, García-Martín A, Saborido A, de la Mata I, Arroyo M (2019) Biochemical properties and biotechnological applications of microbial enzymes involved in the degradation of polyester-type plastics. Biochim Biophys Acta Proteins Proteom 1868:140315. https://doi.org/10.1016/j.bbapap.2019.140315
Lucas N, Bienaime C, Belloy c, Queneudec M, Silvestre F, Nava-Saucedo JE, (2008) Polymer biodegradation: Mechanisms and estimation techniques. Chemosphere 73:429–442. https://doi.org/10.1016/j.chemosphere.2008.06.064
Ong SY, Chee JY, Sudesh K (2017) Degradation of polyhydroxyalkanoate (PHA): a review. J Sib Fed Univ Biol 10:211–225
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1503–1555
Amara AA, Moawad H (2011) PhaC synthases and PHA depolymerases: The enzymes that produce and degrade plastic. IIUM Engineering Journal 12:21–37
Tokiwa Y, Calabia BP (2004) Review degradation of microbial polyesters. Biotechnol Lett 26:1181–1189. https://doi.org/10.1023/B:BILE.0000036599.15302.e5
Sharma PK, Mohanan N, Sidhu R, Levin DB (2019) Colonization and degradation of polyhydroxyalkanoates by lipase-producing bacteria. Can J Microbiol 65:461–475. https://doi.org/10.1139/cjm-2019-0042
Vandi LJ, Chan CM, Werker A, Richardson D, Laycock B, Pratt S (2018) Wood-PHA composites: Mapping opportunities Polymers 10(7):751. https://doi.org/10.3390/polym10070751
Pratt S, Vandi LJ, Gapes D, Werker A, Oehmen A, Laycock B (2019) Polyhydroxyalkanoate (PHA) Bioplastics from Organic Waste. In: Bastidas-Oyanedel JR, Schmidt J (eds) Biorefinery. Springer, Cham
Shogren R, Wood D, Orts W, Glenn G (2019) Plant-based materials and transitioning to a circular economy. Sustain Prod Consum 19:194–215. https://doi.org/10.1016/j.spc.2019.04.007
Research and Markets (2021) The Global Market for Polyhydroxyalkanoates (PHA) to 2030. https://www.researchandmarkets.com/reports/5437951. Accessed 18 Jan 2022.
Moshood TD, Nawanir G, Mahmud F, Mohamad F, Ahmad MH, Ghani AA (2022) Biodegradable plastic applications towards sustainability: A recent innovations in the green product. Cleaner Eng Technol 6:100404. https://doi.org/10.1016/j.clet.2022.100404
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446. https://doi.org/10.1039/B812677C
Brigham CJ, Riedel SL (2018) The potential of polyhydroxyalkanoate production from food wastes. Appl Food Biotechnol 6:7–18
Goh QH, Farouk AA, Chew IL (2022) Optimizing the bioplastic chemical building block with wastewater sludge as the feedstock using carbon-hydrogen-oxygen framework RCR. Advances 176:105920. https://doi.org/10.1016/j.resconrec.2021.105920
Shahzad K, Rehan M, Rashid MI, Ali N, Summan AS, Ismail IMI (2022) Sustainability evaluation of polyhydroxyalkanoate production from slaughterhouse residues utilising emergy accounting. Polymers 14(1):118. https://doi.org/10.3390/polym14010118
Wang K, Hobby AM, Chen Y, Chio A, Jenkins BM, Zhang R (2022) Techno-economic analysis on an industrial-scale production system of polyhydroxyalkanoates (PHA) from cheese by-products by halophiles. Processes 10(1):17. https://doi.org/10.3390/pr10010017
Favaro L, Basaglia M, Casella S (2018) Improving polyhydroxyalkanoate production from inexpensive carbon sources by genetic approaches: a review. Biofuels Bioprod Biorefin 13:208–227. https://doi.org/10.1002/bbb.1944
El-malek FA, Khairy H, Farag A, Omar S (2020) The sustainability of microbial bioplastics, production and applications. Int J Biol Macromol 157:319–328. https://doi.org/10.1016/j.ijbiomac.2020.04.076
Pradhan S, Dikshit PK, Moholkar VS (2020) Production, characterization, and applications of biodegradable polymer: polyhydroxyalkanoates. In: Katiyar V, Kumar A, Mulchandani N (eds) Advances in Sustainable Polymers: Synthesis. Fabrication and Characterization Springer Nature, Singapore
Pavan FA, Junqueira TL, Watanabe MD, Bonomi A, Quines LK, Schmidell W, De Aragao GM (2019) Economic analysis of polyhydroxybutyrate production by Cupriavidus necator using different routes for product recovery. Biochem Eng J 146:97–104. https://doi.org/10.1016/j.bej.2019.03.009
Sodhi AS, Sharma N, Bhatia S, Verma A, Soni S, Batra N (2022) Insights on sustainable approaches for production and applications of value added products. Chemosphere 286:131623. https://doi.org/10.1016/j.chemosphere.2021.131623
Anjali M, Sukumar C, Kanakalakshmi A, Shanthi K (2014) Enhancement of growth and production of polyhydroxyalkanoates by Bacillus subtilis from agro-industrial waste as carbon substrates. Compos Interfaces 21:111–119. https://doi.org/10.1080/15685543.2013.834200
Rathika R, Janaki V, Shanthi K, Kamala-Kannan S (2019) Bioconversion of agro-industrial effluents for polyhydroxyalkanoates production using Bacillus subtilis RS1. Int J Environ Sci Technol 16:5725–5734. https://doi.org/10.1007/s13762-018-2155-3
Suwannasing W, Imai T, Kaewkannetra P (2015) Cost-effective defined medium for the production of polyhydroxyalkanoates using agricultural raw materials. Bioresour Technol 194:67–74. https://doi.org/10.1016/j.biortech.2015.06.087
Bozorg A, Vossoughi M, Kazemi A, Alemzadeh I (2015) Optimal medium composition to enhance poly-β-hydroxybutyrate production by Ralstonia eutropha using cane molasses as sole carbon source. Appl Food Biotechnol 2:39–47
Ray S, Prajapati V, Patel K, Trivedi U (2016) Optimization and characterization of PHA from isolate Pannonibacter phragmitetus ERC8 using glycerol waste. Int J Biol Macromol 86:741–749. https://doi.org/10.1016/j.ijbiomac.2016.02.002
Sallau AB, Salim B, Salihu A (2018) assessment of bioplastic producing potential of Bacillus subtilis using some agricultural residues as carbon source. Sci J Univ Zakho 6:42–45
Yousuf RG, Winterburn JB (2017) Waste date seed oil extract as an alternative feedstock for Poly (3-hydroxybutyrate) synthesis. Biochem Eng J 127:68–76. https://doi.org/10.1016/j.bej.2017.08.007
Amini M, Yousefi-Massumabad H, Younesi H, Abyar H, Bahramifar N (2020) Production of the polyhydroxyalkanoate biopolymer by Cupriavidus necator using beer brewery wastewater containing maltose as a primary carbon source. J Environ Chem Eng 8:103588. https://doi.org/10.1016/j.jece.2019.103588
Devi NC, Mazumder PB, Bhattacharjee A (2018) Statistical optimization of polyhydroxybutyrate production by Bacillus pumilus H9 using cow dung as a cheap carbon source by response surface methodology. J Polym Environ 26:3159–3167. https://doi.org/10.1007/s10924-018-1194-7
Bose SA, Raja S, Jeyaram K, Arockiasamy S, Velmurugan S (2020) Investigation of fermentation condition for production enhancement of polyhydroxyalkanoate from cheese whey by Pseudomonas sp. J Microbiol Biotech Food Sci 9:890
Getachew A, Woldesenbet F (2016) Production of biodegradable plastic by polyhydroxybutyrate (PHB) accumulating bacteria using low cost agricultural waste material. BMC Res Notes 9:509. https://doi.org/10.1186/s13104-016-2321-y
Hong YG, Moon YM, Hong JW, Choi TR, Jung HR, Yang SY, Jang DW, Park YR, Brigham C, Kim JS, Lee YK, Yang YH (2019) Discarded egg yolk as an alternate source of Poly(3-Hydroxybutyrate-co-3-hydroxyhexanoate). J Microbiol Biotechnol 29:382–391. https://doi.org/10.4014/jmb.1811.11028
Chanasit W, Hodgson B, Sudesh K, Umsakul K (2016) Efficient production of polyhydroxyalkanoates (PHAs) from Pseudomonas mendocina PSU using a biodiesel liquid waste (BLW) as the sole carbon source. Biosci Biotechnol Biochem 80:1440–1450. https://doi.org/10.1080/09168451.2016.1158628
Flores-Sánchez A, López-Cuellar M, Pérez-Guevara F, Figueroa López U, Martín-Bufájer JM, Vergara-Porras B (2017) Synthesis of poly-(R-hydroxyalkanoates) by Cupriavidus necator ATCC 17699 using mexican avocado (Persea americana) oil as a carbon source. Int J Polym Sci 2017:1–10. https://doi.org/10.1155/2017/6942950
Penkhrue W, Jendrossek D, Khanongnuch C, Pathom-aree W, Aizawa T, Behrens RL, Lumyong S (2020) Response surface method for polyhydroxybutyrate (PHB) bioplastic accumulation in Bacillus drentensis BP17 using pineapple peel. PLoS ONE 15(3):e0230443. https://doi.org/10.1371/journal.pone.0230443
Al-Battashi H, Annamalai N, Al-Kindi S, Nair AS, Al-Bahry S, Verma JP, Sivakumar N (2019) Production of bioplastic (poly-3-hydroxybutyrate) using wastepaper as a feedstock: Optimization of enzymatic hydrolysis and fermentation employing Burkholderia sacchari. J Clean Prod 214:236–247. https://doi.org/10.1016/j.jclepro.2018.12.239
Dalsasso RR, Pavan FA, Bordignon SE, de Aragão GMF, Poletto P (2019) Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem 85:12–18. https://doi.org/10.1016/j.procbio.2019.07.007
Hassan MA, Bakhiet EK, Hussein HR, Ali SG (2019) Statistical optimization studies for polyhydroxybutyrate (PHB) production by novel Bacillus subtilis using agricultural and industrial wastes. Int J Environ Sci Technol 16:3497–3512. https://doi.org/10.1007/s13762-018-1900-y
Kynadi AS, Suchithra TV (2017) Rubber seed oil as a novel substrate for polyhydroxyalkanoates accumulation in Bacillus cereus. Clean: Soil, Air, Water 45:1600572. https://doi.org/10.1002/clen.201600572
Sangkharak K, Khaithongkaeo P, Chuaikhunupakarn T, Choonut A, Prasertsan P (2020) The production of polyhydroxyalkanoate from waste cooking oil and its application in biofuel production. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-00657-6
Sangkharak K, Paichid N, Yunu T, Klomklao S, Prasertsan P (2020) Utilisation of tuna condensate waste from the canning industry as a novel substrate for polyhydroxyalkanoate production. Biomass Conv Bioref. https://doi.org/10.1007/s13399-019-00581-4
Vega-Castro O, Contreras-Calderon J, León E, Segura A, Arias M, Pérez L, Sobral PJA (2016) Characterization of a polyhydroxyalkanoate obtained from pineapple peel waste using Ralsthonia eutropha. J Biotechnol 231:232–238. https://doi.org/10.1016/j.jbiotec.2016.06.018
Vega-Castro O, León E, Arias M, Cesario MT, Ferreira F, da Fonseca MMR, Segura A, Valencia P, Simpson R, Nuñez H, Contreras-Calderon J (2021) Characterization and production of a polyhydroxyalkanoate from cassava peel waste: Manufacture of biopolymer microfibers by electrospinning. J Polym Environ 29:187–200. https://doi.org/10.1007/s10924-020-01861-1
Kourmentza C, Plácido J, Venetsaneas N, Burniol-Figols A, Varrone C, Gavala HN, Reis MA (2017) Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 4:55. https://doi.org/10.3390/bioengineering4020055
Vu DH, Åkesson D, Taherzadeh MJ, Ferreira JA (2020) Recycling strategies for polyhydroxyalkanoate-based waste materials: An overview. Bioresour Technol 298:122393. https://doi.org/10.1016/j.biortech.2019.122393
Macagnan KL, Alves MI, Moreira AS (2019) Approaches for enhancing extraction of bacterial polyhydroxyalkanoates for industrial applications. In: Kalia V (ed) Biotechnological Applications of Polyhydroxyalkanoates. Springer, Singapore
Pérez-Rivero C, López-Gómez JP, Roy I (2019) A sustainable approach for the downstream processing of bacterial polyhydroxyalkanoates: State-of-the-art and latest developments. Biochem Eng J 150:107283. https://doi.org/10.1016/j.bej.2019.107283
Jiang G, Johnston B, Townrow DE, Radecka I, Koller M, Chaber P, Adamus G, Kowalczuk M (2018) Biomass extraction using non-chlorinated solvents for biocompatibility improvement of polyhydroxyalkanoates. Polymers 10:731. https://doi.org/10.3390/polym10070731
Kunasundari B, Sudesh K (2011) Isolation and recovery of microbial polyhydroxyalkanoates. EXPRESS Polym Lett 5:620–634. https://doi.org/10.3144/expresspolymlett.2011.60
Tripathi AD, Joshi T, Khosravi-Darani K, Koller M, Singh SP, Shrivastava A, Mishra S (2016) Recovery and characterization of polyhydroxyalkanoates. Recent Biotechnol Adv 2:267–303. https://doi.org/10.2174/9781681083735116020008
Gamero JER, Favaro L, Pizzocchero V, Lomolino G, Basaglia M, Casella S (2018) Nuclease expression in efficient polyhydroxyalkanoates-producing bacteria could yield cost reduction during downstream processing. Bioresour Technol 261:176–181. https://doi.org/10.1016/j.biortech.2018.04.021
Jaffe M, Ophir Z, Pai V (2003) Biorelevant characterization of biopolymers. Thermochim Acta 396:141–152. https://doi.org/10.1016/S0040-6031(02)00524-5
Tomoda BT, Yassue-Cordeiro PH, Ernesto JV, Lopes PS, Péres LO, Silva CF, Moraes MA (2020) In: Matthew D (ed) Biopolymer Membranes and Films. SPi Global. Elsevier, India.
Mukherjee S, Gowen A (2015) A review of recent trends in polymer characterization using non-destructive vibrational spectroscopic modalities and chemical imaging. Anal Chim Acta 895:12–34. https://doi.org/10.1016/j.aca.2015.09.006
Udayakumar GP, Muthusamy S, Selvaganesh B, Sivarajasekar N, Rambabu K, Banat F, Sivamani S, Sivakumar N, Hosseini-Bandegharaei A, Show PL (2021) Biopolymers and composites: Properties, characterization and their applications in food, medical and pharmaceutical industries. J Environ Chem Eng 9:105322. https://doi.org/10.1016/j.jece.2021.105322
Godbole S (2016) Methods for identification, quantification and characterization of polyhydroxyalkanoates-a review. Int J Bioassays 5:4977–4983
Hong K, Sun S, Tian W, Chen GQ, Huang W (1999) A rapid method for detecting bacterial polyhydroxyalkanoates in intact cells by Fourier transform infrared spectroscopy. Appl Microbiol Biotechnol 51:523–526. https://doi.org/10.1007/s002530051427
Stanley A, Murthy PSK, Vijayendra SVN (2020) Characterization of polyhydroxyalkanoate produced by Halomonas venusta KT832796. J Polym Environ 28:973–983. https://doi.org/10.1007/s10924-020-01662-6
Mohanrasu K, Rao RGR, Dinesh GH, Zhang K, Prakash GS, Song DP, Muniyasamy S, Pugazhendhi A, Jeyakanthan J, Arun A (2020) Optimization of media components and culture conditions for polyhydroxyalkanoates production by Bacillus megaterium. Fuel 271:117522. https://doi.org/10.1016/j.fuel.2020.117522
Ojha N, Das N (2020) Process optimization and characterization of polyhydroxyalkanoate copolymers produced by marine Pichia kudriavzevii VIT-NN02 using banana peels and chicken feather hydrolysate. Biocatal Agric Biotechnol 27:101616. https://doi.org/10.1016/j.bcab.2020.101616
Evora MC, Gonçalez OL, Dutra RC, Diniz MF, Wiebeck H, Silva LG (2002) A comparison of transmission, reflection and photoacoustic FTIR techniques in the analysis of recycled and irradiated polyamide-6. Polímeros 12:60–68. https://doi.org/10.1590/S0104-14282002000100013
Kamnev AA, Tugarova AV, Dyatlova YA, Tarantilis PA, Grigoryeva OP, Fainleib AM, De Luca S (2018) Methodological effects in Fourier Transform Infrared (FTIR) spectroscopy: Implications for structural analyses of biomacromolecular samples. Spectrochim Acta A 193:558–564. https://doi.org/10.1016/j.saa.2017.12.051
Ricci A, Olejar KJ, Parpinello GP, Kilmartin PA, Versari A (2015) Application of Fourier Transform Infrared (FTIR) spectroscopy in the characterization of tannins. Appl Spectrosc Rev 50:407–442. https://doi.org/10.1080/05704928.2014.1000461
Arumugam A, Shereen MF (2019) Bioconversion of Calophyllum inophyllum oilcake for intensification of rhamnolipid and polyhydroxyalkanoates co-production by Enterobacter aerogenes. Bioresour Technol 296:122321. https://doi.org/10.1016/j.biortech.2019.122321
Sabapathy PC, Devaraj S, Parthiban A, Pugazhendhi A, Kathirvel P (2019) Aegle marmelos: A novel low cost substrate for the synthesis of polyhydroxyalkanoate by Bacillus aerophilus RSL-7. Biocatal Agric Biotechnol 18:101021. https://doi.org/10.1016/j.bcab.2019.101021
Kansiz M, Domínguez-Vidal A, Mcnaughton D, Lendl B (2007) Fourier-transform infrared (FTIR) spectroscopy for monitoring and determining the degree of crystallisation of polyhydroxyalkanoates (PHAs). Anal Bioanal Chem 388:1207–1213. https://doi.org/10.1007/s00216-007-1337-5
Tapadiya A, Vasanthan N (2017) Crystallization and alkaline hydrolysis of poly(3-hydroxybutyrate) films probed by thermal analysis and infrared spectroscopy. Int J Biol Macromol 102:1130–1137. https://doi.org/10.1016/j.ijbiomac.2017.04.095
Duemichen E, Eisentraut P, Celina M, Braun U (2019) Automated thermal extraction-desorption gas chromatography mass spectrometry: A multifunctional tool for comprehensive characterization of polymers and their degradation products. J Chromatogr A 1592:133–142. https://doi.org/10.1016/j.chroma.2019.01.033
Silva F, Matos M, Pereira b, Ralo C, Pequito D, Marques N, Carvalho G, Reis MAM, (2022) An integrated process for mixed culture production of 3-hydroxyhexanoate-rich polyhydroxyalkanoates from fruit waste. Chem Eng J 427:131908. https://doi.org/10.1016/j.cej.2021.131908
Ge L, Tan GYA, Wang L, Chen CL, Li L, Tan SN, Wang JY (2016) Determination of monomeric composition in polyhydroxyalkanoates by liquid chromatography coupled with on-line mass spectrometry and off-line nuclear magnetic resonance. Talanta 146:107–113. https://doi.org/10.1016/j.talanta.2015.08.029
Khang TU, Kim MJ, Yoo JI, Sohn YJ, Jeon SG, Park SJ, Na JG (2021) Rapid analysis of polyhydroxyalkanoate contents and its monomer compositions by pyrolysis-gas chromatography combined with mass spectrometry (Py-GC/MS). Int J Biol Macromol 174:449–456. https://doi.org/10.1016/j.ijbiomac.2021.01.108
Maheshwari N, Kumar M, Thakur IS, Srivastava S (2018) Production, process optimization and molecular characterization of polyhydroxyalkanoate (PHA) by CO2 sequestering B. cereus SS105. Bioresour Technol 254:75–82. https://doi.org/10.1016/j.biortech.2018.01.002
Wagle AR, Dixit YM, Vakil BV (2019) Scale up studies for polyhydroxyalkanoate production by a Bacillus flexus strain with industrial potential. Indian J Microbiol 59:383–386. https://doi.org/10.1007/s12088-019-00807-z
Tanaka S (2021) Recent advances in dynamic nuclear polarization-enhanced NMR spectroscopy for organic polymers. Annual Reports on NMR Spectroscopy. Elsevier, Amsterdam
Besghini D, Mauri M, Simonutti R (2019) Time domain NMR in polymer science: From the laboratory to the industry. Appl Sci 9:1801. https://doi.org/10.3390/app9091801
Macomber RS (1998) A complete introduction to modern NMR spectroscopy. Wiley, New York
Spiess HW (2017) 50th anniversary perspective: The importance of NMR spectroscopy to macromolecular science. Macromolecules 50:1761–1777. https://doi.org/10.1021/acs.macromol.6b02736
De Graaf RA (2019) In vivo NMR spectroscopy: principles and techniques. Wiley, New York
Olatunji OO et al (2018) Thermo gravimetric characterization of biomass properties: A review. IOP Conf Ser Mater Sci Eng 423:012175
Seifi H, Gholami T, Seifi S, Ghoreishi SM, Salavati-Niasari M (2020) A review on current trends in thermal analysis and hyphenated techniques in the investigation of physical, mechanical and chemical properties of nanomaterials. J Anal Appl Pyrolysis 149:104840. https://doi.org/10.1016/j.jaap.2020.104840
Saadatkhah N, Carillo Garcia A, Ackermann S, Leclerc P, Latifi M, Samih S, Patience GS, Chaouki J (2020) Experimental methods in chemical engineering: Thermogravimetric analysis-TGA. Can J Chem Eng 98:34–43. https://doi.org/10.1002/cjce.23673
Al-Salem SM (ed) (2019) Plastics to Energy, 1st edn. Cambridge, William Andrew
Huseynov EM, Naghiyev TG, Aliyeva US (2020) Thermal parameters investigation of neutron-irradiated nanocrystalline silicon carbide (3C-SiC) using DTA, TGA and DTG methods. Phys Rev B Condens Matter 577:411788. https://doi.org/10.1016/j.physb.2019.411788
Khoshooei MA, Fazlollahi F, Maham Y (2019) A review on the application of differential scanning calorimetry (DSC) to petroleum products. J Therm Anal Calorim 138:3455–3484. https://doi.org/10.1007/s10973-019-08244-2
Gharanjig H, Gharanjig K, Hosseinnezhad M, Jafari SM (2020) Introduction to characterization of nanoencapsulated food ingredients. In: Jafari MS (ed) Characterization of Nanoencapsulated Food Ingredients, 1st edn. Academic Press, London
Müller AJ, Mitchell RM (2016) In: Guo Q (ed.) Polymer Morphology: Principles, Characterization, and Processing, 1st edn. Wiley, New Jersey.
Drzeżdżon J, Jacewicz D, Sielicka A, Chmurzyński L (2019) Characterization of polymers based on differential scanning calorimetry based techniques. Trends Anal Chem 110:51–56. https://doi.org/10.1016/j.trac.2018.10.037
Gaisford S, Kett V, Haines P (eds) (2016) Principles of Thermal Analysis and Calorimetry, 2nd edn. Royal Society of Chemistry, London
Joyline M, Aruna K (2019) Production and characterization of polyhydroxyalkanoates (PHA) by Bacillus megaterium strain JHA using inexpensive agro-industrial wastes. Int J Recent Sci Res 10:33359–33374
Ausejo JG, Rydz J, Musioł M, Sikorska W, Sobota M, Włodarczyk J, Adamus G, Janeczek H, Kwiecień I, Hercog A, Johnston B, Khan HR, Kannappan V, Jones KR, Morris MR, Jiang G, Radecka I, Kowalczuk M (2018) A comparative study of three-dimensional printing directions: The degradation and toxicological profile of a PLA/PHA blend. Polym Degrad Stab 152:191–207. https://doi.org/10.1016/j.polymdegradstab.2018.04.024
Ecker JV, Burzic I, Haider A, Hild S, Rennhofer H (2019) Improving the impact strength of PLA and its blends with PHA in fused layer modelling. Polym Test 78:105929. https://doi.org/10.1016/j.polymertesting.2019.105929
Lee EH, Jee MH, Kang CS, Baik DH (2019) Preparation and characterization of polyhydroxyamide hybrid nanocomposite films containing MWCNTs and clay as reinforcing materials. Fibers Polym 20:832–838. https://doi.org/10.1007/s12221-019-1034-y
Lukasiewicz B, Basnett P, Nigmatullin R, Matharu R, Knowles JC, Roy I (2018) Binary polyhydroxyalkanoate systems for soft tissue engineering. Acta Biomater 71:225–234. https://doi.org/10.1016/j.actbio.2018.02.027
Al-Mutairi NH, Mousa ZO (2021) Some methodes for measurements of polymer degradation: A review. J Univ Babylon Eng Sci 29:99–114
Fair RA, Xie R, Lee Y, Colby RH, Gomez ED (2021) Molecular weight characterization of conjugated polymers through gel permeation chromatography and static light scattering. ACS Appl Polym 3:4572–4578. https://doi.org/10.1021/acsapm.1c00647
Ugbolue SCO (2017) In: Ugbolue SCO (ed) Polyolefin Fibres 2nd edn. Woodhead Publishing, Sawston.
Ma J, Sun G, Sun D, Yu F, Hu M, Lu T (2021) Application of gel permeation chromatography technology in asphalt materials: A review. Constr Build Mater 278:122386. https://doi.org/10.1016/j.conbuildmat.2021.122386
Akhtar K, Khan SA, Khan SB, Asiri AM (2018) Scanning electron microscopy: principle and applications in nanomaterials characterization. In: Sharma S (ed) Handbook of Materials Characterization, Springer. Cham, New York
Ul-Hamid A (2018) A Beginners’ Guide to Scanning Electron Microscopy. Springer Nature, Basingstoke
Samrot AV, Samanvitha SK, Shobana N, Renitta ER, Senthil Kumar P, Kumar SS, Abirami S, Dhiva S, Bavanilatha M, Prakash P, Saigeetha S, Shree KS, Thirumurugan R (2021) The synthesis, characterization and applications of polyhydroxyalkanoates (PHAs) and PHA-based nanoparticles. Polymers 13(19):3302. https://doi.org/10.3390/polym13193302
Fialová D, Skoupý R, Drozdová E, Paták A, Piňos J, Šín L, Beňuš R, Klíma B (2017) The application of scanning electron microscopy with energy-dispersive X-ray spectroscopy (SEM-EDX) in ancient dental calculus for the reconstruction of human habits. Microsc Microanal 23:1207–1213. https://doi.org/10.1017/S1431927617012661
Funding
The authors acknowledge the Coordination for the Improvement of Higher Education Personnel (CAPES) under Finance Code 001 and the Banco do Nordeste do Brasil - BNB/FUNDECI (Grant ETENE/FUNDECI 01/2015) for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare no financial or personal conflicts of interest influencing the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guimarães, T.C., Araújo, E.S., Hernández-Macedo, M.L. et al. Polyhydroxyalkanoates: Biosynthesis from Alternative Carbon Sources and Analytic Methods: A Short Review. J Polym Environ 30, 2669–2684 (2022). https://doi.org/10.1007/s10924-022-02403-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-022-02403-7