Abstract
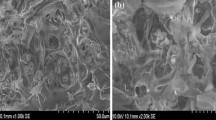
Calligonum crinitum, a desert plant, was used as a natural adsorbent for the removal of Pb(II) ions from aqueous solutions. The sorption capacity of the sorbent was investigated through batch adsorption, as a function of contact time, metal ion concentration, and pH. The surface chemistry of the sorbent was probed using Fourier transform infrared spectroscopy, allowing an understanding of the key functionalities involved in binding the Pb(II) ions to the sorbent. Textural characteristics, including surface area and pore volume, showed higher surface areas relative to other natural adsorbents previously reported. Surface morphological analysis, observed via Scanning Electron Microscopy images and Energy-Dispersive X-Ray analysis, confirmed adsorption of Pb(II) ions, up to 71% on the surface of calligonum crinitum. Isothermal analysis, using Langmuir, Freundlich and Temkin models with nonlinear regression, showedthe Langmuir model to best represent adsorption within this system, yielding a maximum adsorption capacity of 337 mg g−1 (pH 5.5, 30 °C). The optimum conditions of adsorption was pH, 5.5; adsorbent concentration, 0.3 g/l, contact time, 20 min at 30 °C. The Freundlich constant of < 10 indicates that adsorption of Pb(II) ions is favourable onto calligonum crinitum. Adsorption kinetics were modelled using pseudo-first-order, pseudo-second-order and intra-particle diffusion models; the results indicate that the kinetics are pseudo-second-order, suggesting a degree of chemical influence in the adsorption process. While thermodynamic analysis shows the process to be exothermic in nature with an ordering of solute within the adsorption process. Calligonum crinitum has, therefore, been proven as an effective sorbent for the removal of Pb(II) ions from aqueous solutions, with potential in water remediation processes.










Similar content being viewed by others
References
Barakat M (2011) New trends in removing heavy metals from industrial wastewater. Arabian J Chem 4(4):361–377
Dong L, Zhu Z, Qiu Y, Zhao J (2010) Removal of lead from aqueous solution by hydroxyapatite/magnetite composite adsorbent. Chem Eng J 165(3):827–834
Needleman H (2004) Lead poisoning. Annu Rev Med 55:209–222
World Health Organization (2010) Childhood lead poisoning, p 72
Brooks RM, Bahadory M, Tovia F, Rostami H (2010) Removal of lead from contaminated water. Int J Soil, Sediment Water 3(2):14
Arbabi M, Hemati S, Amiri M (2015) Removal of lead ions from industrial wastewater: a review of removal methods. Int J Epidemiol Res 2(2):105–109
Abdel-Ghani N, Hefny M, El-Chaghaby GA (2007) Removal of lead from aqueous solution using low cost abundantly available adsorbents. Int J Environ Sci Technol 4(1):67–73
Abdel-Halim S, Shehata A, El-Shahat M (2003) Removal of lead ions from industrial waste water by different types of natural materials. Water Res 37(7):1678–1683
Faisal AA, Nassir ZS, Naji LA, Naushad M, Ahamad T (2020) A sustainable approach to utilize olive pips for the sorption of lead ions: numerical modeling with aid of artificial neural network. Sustainable Chem Pharm 15:100220
Singha B, Naiya TK, Kumar Bhattacharya A, Das SK (2011) Cr (VI) ions removal from aqueous solutions using natural adsorbents–FTIR studies. J Environ Prot 2(06):729
Salem A, Sene RA (2011) Removal of lead from solution by combination of natural zeolite–kaolin–bentonite as a new low-cost adsorbent. Chem Eng J 174(2–3):619–628
Hashem A, Hammad HA, Al-Anwar A (2016) Modified Camelorum tree particles as a new adsorbent for adsorption of Hg (II) from aqueous solutions: kinetics, thermodynamics and non-linear isotherms. Desalin Water Treat 57(50):23827–23843
Hashem A, Badawy SM (2015) Sesbania sesban L. biomass as a novel adsorbent for removal of Pb (II) ions from aqueous solution: non-linear and error analysis. Green Process Synthe 4(3):179–190
Baig T, Garcia A, Tiemann K, Gardea-Torresdey J (1999) Adsorption of heavy metal ions by the biomass of Solanum elaeagnifolium (Silverleaf night-shade). In: Proceedings of the 1999 conference on Hazardous Waste Research. Regal Riverfront Hotel, St. Louis, Missouri
Edokpayi JN, Odiyo JO, Msagati TA, Popoola EO (2015) A novel approach for the removal of lead (II) ion from wastewater using mucilaginous leaves of diceriocaryum eriocarpum plant. Sustainability 7(10):14026–14041
Hashem A, Al-Anwar A, Nagy NM, Hussein DM, Eisa S (2016) Isotherms and kinetic studies on adsorption of Hg (II) ions onto Ziziphus spina-christi L. from aqueous solutions. Green Process Synth 5(2):213–224
Cornelissen J, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich D, Reich P, Ter Steege H, Morgan H, Van Der Heijden M (2003) A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51(4):335–380
Malandrino M, Abollino O, Giacomino A, Aceto M, Mentasti E (2006) Adsorption of heavy metals on vermiculite: influence of pH and organic ligands. J Colloid Interface Sci 299(2):537–546
Kannan N, Sundaram MM (2001a) Kinetics and mechanism of removal of methylene blue by adsorption on various carbons—a comparative study. Dyes Pigm 51(1):25–40
Kapoor A, Yang R (1989) Correlation of equilibrium adsorption data of condensible vapours on porous adsorbents. Gas Sep Purif 3(4):187–192
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. Part I. Solids. J Am Chem Soc 38(11):2221–2295
Freundlich H (1907) Über die adsorption in lösungen. Zeitschrift für physikalische Chemie 57(1):385–470
Temkin M (1940) Kinetics of ammonia synthesis on promoted iron catalysts. Acta physiochim URSS 12:327–356
Lagergren SK (1898) About the theory of so-called adsorption of soluble substances. Sven Vetenskapsakad Handingarl 24:1–39
Ho YS, McKay G (2000) The kinetics of sorption of divalent metal ions onto sphagnum moss peat. Water Res 34:735–742
Weber WJ, Morris JC (1963) Kinetics of adsorption on carbon from solution. J Sanitary Eng Div 89(2):31–60
Thommes M, Kaneko K, Neimark AV, Olivier JP, Rodriguez-Reinoso F, Rouquerol J, Sing KS (2015) Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC technical report). Pure Appl Chem 87(9–10):1051–1069
Hu M, Tian H, He J (2019) Unprecedented selectivity and rapid uptake of CuS nanostructures toward Hg (II) ions. ACS Appl Mater Interfaces 11(21):19200–19206
Hashem A, Fletcher A, El-Sakhawy M, Mohamed LA, Farag S (2020) Aminated hydroximoyl camelthorn residues as a novel adsorbent for extracting Hg (II) from contaminated water: studies of isotherm, kinetics, and mechanism. J Polym Environ 28:1–13
Hashema A, Fletcher AJ, Younis H, Mauof H, Abou-Okeil A (2020) Adsorption of Pb(II) ions from contaminated water by 1,2,3,4-butanetetracarboxylic acid-modified microcrystalline cellulose: Isotherms, kinetics, and thermodynamic studies. Int J Biol Macromol 164:3193–3203
Hashem A, Hammad HA, Al-Anwar A (2015) Chemically modified Retama raetam biomass as a new adsorbent for Pb (II) ions from aqueous solution: non-linear regression, kinetics and thermodynamics. Green Process Synth 4(6):463–478
Tütem E, Apak R, Ünal ÇF (1998) Adsorptive removal of chlorophenols from water by bituminous shale. Water Res 32(8):2315–2324
Kannan N, Sundaram MM (2001b) Kinetics and mechanism of removal of methylene blue adsorption on various carbons—a comparative study. Dyes Pigm 51(1):25–40
Poots V, McKay G, Healy J (1978) Removal of basic dye from effluent using wood as an adsorbent. J Water Pollut Control Fed 50:926–935
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development, and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag, a low-cost adsorbent. Ind Eng Chem Res 37(1):192–202
Vazquez G, Gonzalez-Alvarez J, Freire S, López-Lorenzo M, Antorrena G (2002) Removal of cadmium and mercury ions from aqueous solution by sorption on treated Pinus pinaster bark: kinetics and isotherms. Biores Technol 82(3):247–251
Yu LJ, Shukla SS, Dorris KL, Shukla A, Margrave J (2003) Adsorption of chromium from aqueous solutions by maple sawdust. J Hazard Mater 100(1–3):53–63
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safri, A., Fletcher, A.J., Abdel-Halim, E. et al. Calligonum Crinitum as a Novel Sorbent for Sorption of Pb(II) from Aqueous Solutions: Thermodynamics, Kinetics, and Isotherms. J Polym Environ 29, 1505–1515 (2021). https://doi.org/10.1007/s10924-020-01975-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-020-01975-6