Abstract
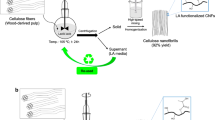
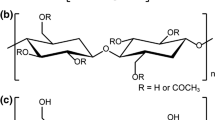
Highly crystalline cellulose nanofibers with a high density of carboxylate groups only on the surfaces were prepared from both softwood and non-wood cellulose pulp. The preparation method used 2,2,6,6-tetramethylpiperidine-1-oxyl (TEMPO)-mediated oxidation of native cellulose fibrils in an aqueous TEMPO/NaBr/NaClO system and subsequent postoxidation with NaClO2 in acetate buffer (pH 4.8). The TEMPO-oxidized cellulose nanofibers (TOCNs) possessed a carboxylate content of 1.7 mmol g−1 and a crystallinity of 67–69% with a crystallite size of ∼3 nm. The TOCNs were used to produce highly crystalline TOCN-graft-poly(lactic acid) (PLA) nanocomposites via ring-opening polymerization of l-lactide in a polar aprotic solvent. Effects of the reaction temperature and the molar ratio of l-lactide to carboxylate surface groups, on the efficiency of surface grafting were investigated to potentially improve the crystallinity and thermal properties of the nanocomposites. The crystallinity of TOCN-g-PLA products was 59–66% greater than the crystallinity of neat PLA.










Similar content being viewed by others
Abbreviations
- BBP:
-
Bagasse bleached pulp
- CDA:
-
Cellulose diacetate
- CDA-g-PLAs:
-
Cellulose diacetate-graft-poly(lactic acid)s
- CNC:
-
Cellulose nanocrystals
- Cr :
-
Crystallinity (%)
- CS :
-
Crystallite size (nm)
- DMSO:
-
Dimethyl sulfoxide
- DSC:
-
Differential scanning calorimetry
- DTGA:
-
Derivative thermogravimetric analysis
- I am :
-
Minimum height (i.e. the plateau) between peaks at 200 and 110 planes
- I 200 :
-
Intensity of diffraction peak at 200 plane of crystalline contribution
- K :
-
Dimensionless crystal shape factor
- LA:
-
l-lactide
- N :
-
Molarity (M) of NaOH solution
- NMR:
-
Nuclear magnetic resonance
- PTFE:
-
Polytetrafluoroethylene
- PLA:
-
Polylactic acid
- Q P1 :
-
Production rate of carbonyl groups (C=O) through ester formation (h−1)
- Q P2 :
-
Production rate of carbonyl groups (C=O) through PLA formation (h−1)
- Q s :
-
Consumption rate of carboxylate groups (COO−) of TOCN–COOLi (h−1)
- R :
-
Molar ratio of l-lactide to carboxylate content of TOCNs
- ROP:
-
Ring-opening polymerization
- SBKP:
-
Softwood bleached kraft pulp
- SD:
-
Standard deviation
- SEM:
-
Scanning electron microscopy
- T deg :
-
Degradation temperature
- TEMPO:
-
2,2,6,6-Tetramethylpiperidine-1-oxyl
- T g :
-
Glass transition temperature
- T m :
-
Melting temperature
- TGA:
-
Thermogravimetric analysis
- TOCNs:
-
TEMPO-oxidized cellulose nanofibrils
- TOCNs–COONa:
-
TOCNs sodium salts
- TOCNs–COOLi:
-
TOCNs lithium salts
- TOCN-g-PLA:
-
TEMPO-oxidized cellulose nanofibers-graft-poly(lactic acid)s
- V :
-
Volume (mL) of alkali consumed
- W :
-
Mass (g) of dried TOCNs–COONa sample
- Y P1/S :
-
Yield of ester carbonyl groups on carboxylate groups
- Y P2/S :
-
Yield of PLA carbonyl groups on carboxylate groups
- β :
-
Diffraction peak width in radians
- θ :
-
Bragg angle corresponding to the 200 plane
- λ :
-
Wavelength (nm) of the incident X-ray
References
Avinc O, Khoddami A (2009) Overview of poly(lactic acid) (PLA) fibre. Part I: production, properties, performance, environmental impact, and end-use applications of poly(lactic acid) fibres. Fibre Chem 41(6):391–399
Arrieta M, Fortunati E, Dominici F, Rayón E, López J, Kenny J (2014) Multifunctional PLA–PHB/cellulose nanocrystal films: processing, structural and thermal properties. Carbohydr Polym 107:16–24
Fujisawa S, Zhang J, Saito T, Iwata T, Isogai A (2014) Cellulose nanofibrils as templates for the design of poly(L-lactide)-nucleating surfaces. Polymer 55:2937–2942
Wang T, Drzal LT (2012) Cellulose-nanofiber-reinforced poly(lactic acid) composites prepared by a water-based approach. ACS Appl Mater Interfaces 4:5079–5085
Puangsin B, Yang Q, Saito T, Isogai A (2013) Comparative characterization of TEMPO-oxidized cellulose nanofibril films prepared from non-wood resources. Int J Biol Macromol 59:208–213
Fujisawa S, Saito T, Kimura S, Iwata T, Isogai A (2013) Surface engineering of ultrafine cellulose nanofibrils toward polymer nanocomposite materials. Biomacromolecules 14:1541–1546
Chen P-Y, Lian H-Y, Shih Y-F, Chen-Wei S-M (2018) Chemically functionalized plant fibers and carbon nanotubes for high compatibility and reinforcement in polylactic acid (PLA) composite. J Polym Environ 26:1962–1968
Isogai A, Saito T, Fukuzumi H (2011) TEMPO-oxidized cellulose nanofibers. Nanoscale 3:71–85
Kuramae R, Saito T, Isogai A (2014) TEMPO-oxidized cellulose nanofibrils prepared from various plant holocelluloses. React Funct Polym 85:126–133
El-Gendy A, Abou-Zeid RE, Salama A, Diab MA, El-Sakhawy M (2017) TEMPO-oxidized cellulose nanofibers/polylactic acid/TiO2 as antibacterial bionanocomposite for active packaging. Egypt J Chem 60:1007–1014
Miao C, Hamad WY (2016) In-situ polymerized cellulose nanocrystals (CNC)-poly(L-lactide) (PLLA) nanomaterials and applications in nanocomposite processing. Carbohydr Polym 153:549–558
Teramoto Y, Nishio Y (2003) Cellulose diacetate-graft-poly(lactic acid)s: synthesis of wide-ranging compositions and their thermal and mechanical properties. Polymer 44:2701–2709
Mayumi A, Kitaoka T, Wariishi H (2006) Partial substitution of cellulose by ring-opening esterification of cyclic esters in a homogeneous system. J Appl Polym Sci 102:4358–4364
Yu Y, Storti G, Morbidelli M (2009) Ring-opening polymerization of L,L-lactide: kinetic and modeling study. Macromolecules 42:8187–8197
Kaihara S, Matsumura S, Mikos AG, Fisher JP (2007) Synthesis of poly(L-lactide) and polyglycolide by ring-opening polymerization. Nat Protoc 2:2767–2771
Ohya Y, Takamido S, Nagahama K, Ouchi T, Katoono R, Yui N (2009) Polyrotaxane composed of poly-L-lactide and α-cyclodextrin exhibiting protease-triggered hydrolysis. Biomacromolecules 10:2261–2267
Chuensangjun C, Kitaoka T, Chisti Y, Sirisansaneeyakul S (2018) Optimal ring-opening polymerization for producing surface-modified cellulose nanofibers-graft-poly(lactic acid)s. New Biotechnol 44S:S105. Presented at the 18th European Congress on Biotechnology (ECB 2018), Geneva, Switzerland, July 1–4, 2018
Kricheldorf HR, Kreiser-Saunders I, Boettcher C (1995) Polylactones: 31. Sn(II)octoate-initiated polymerization of L-lactide: a mechanistic study. Polymer 36:1253–1259
Fan Y, Saito T, Isogai A (2008) Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 9:192–198
Lizundia E, Vilas JL, León LM (2015) Crystallization, structural relaxation and thermal degradation in poly(L-lactide)/cellulose nanocrystal renewable nanocomposites. Carbohydr Polym 123:256–265
Kobe R, Iwamoto S, Endo T, Yoshitani K, Teramoto Y (2016) Stretchable composite hydrogels incorporating modified cellulose nanofiber with dispersibility and polymerizability: mechanical property control and nanofiber orientation. Polymer 97:480–486
Luiz de Paula E, Roig F, Mas A, Habas J-P, Mano V, Pereira FV, Robin J-J (2016) Effect of surface-grafted cellulose nanocrystals on the thermal and mechanical properties of PLLA based nanocomposites. Eur Polym J 84:173–187
Fujisawa S, Okita Y, Fukuzumi H, Saito T, Isogai A (2011) Preparation and characterization of TEMPO-oxidized cellulose nanofibril films with free carboxyl groups. Carbohydr Polym 84:579–583
Silva Perez D, Montanari S, Vignon MR (2003) TEMPO-mediated oxidation of cellulose III. Biomacromolecules 4:1417–1425
Michael DG, Croteau S, Hazlett JD, Kutowy O (1990) Synthesis and characterization of carboxylated polysulfones. Br Polym J 23:29–39
Bulota M, Tanpichai S, Hughes M, Eichhorn SJ (2012) Micromechanics of TEMPO-oxidized fibrillated cellulose composites. ACS Appl Mater Interfaces 4:331–337
Nikolic L, Ristic I, Adnadjevic B, Nikolic V, Jovanovic J, Stankovic M (2010) Novel microwave-assisted synthesis of poly(D,L-lactide): the influence of monomer/initiator molar ratio on the product properties. Sensors 10:5063–5073
Auras R, Harte B, Selke S (2004) An overview of polylactides as packaging materials. Macromol Biosci 4:835–864
Gonçalves CMB, Coutinho JAP, Marrucho IM (2010) In: Auras R, Lim LT, Selke SEM, Tsuji H (eds) Poly(lactic acid): synthesis, structures, properties, processing, and applications. Wiley, Hoboken, p 97
Kister G, Cassanas G, Vert M (1998) Effects of morphology, conformation and configuration on the IR and Raman spectra of various poly(lactic acid)s. Polymer 39:267–273
Segal L, Creely J, Martin A, Conrad C (1959) An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text Res J 29:786–794
Cao Y, Tan H (2005) Study on crystal structures of enzyme-hydrolyzed cellulosic materials by X-ray diffraction. Enzyme Microb Technol 36:314–317
Saito T, Isogai A (2004) TEMPO-mediated oxidation of native cellulose. The effect of oxidation conditions on chemical and crystal structures of the water-insoluble fractions. Biomacromolecules 5:1983–1989
Shinoda R, Saito T, Okita Y, Isogai A (2012) Relationship between length and degree of polymerization of TEMPO-oxidized cellulose nanofibrils. Biomacromolecules 13:842–849
Saito T, Kimura S, Nishiyama Y, Isogai A (2007) Cellulose nanofibers prepared by tempo-mediated oxidation of native cellulose. Biomacromolecules 8:2485–2491
Stuart B (2004) Infrared spectroscopy: fundamentals and applications. Wiley, Hoboken
He J, Liu Z, Li H-Y, Wang G-H, Pu J-W (2007) Solubility of wood-cellulose in LiCl/DMAC solvent system. For Stud China 9:217–220
Kowalski A, Duda A, Penczek S (2000) Kinetics and mechanism of cyclic esters polymerization initiated with tin(II) octoate. 3. Polymerization of L,L-dilactide. Macromolecules 33:7359–7370
Quynh TM, Mai HH, Lan PN (2013) Stereocomplexation of low molecular weight poly(L-lactic acid) and high molecular weight poly(D-lactic acid), radiation crosslinking PLLA/PDLA stereocomplexes and their characterization. Radiat Phys Chem 83:105–110
Duevel RV, Corn RM (1992) Amide and ester surface attachment reactions for alkanethiol monolayers at gold surfaces as studied by polarization modulation Fourier transform infrared spectroscopy. Anal Chem 64:337–342
Noël M, Fredon E, Mougel E, Masson D, Masson E, Delmotte L (2009) Lactic acid/wood-based composite material. Part 1: synthesis and characterization. Bioresour Technol 100:4711–4716
Monga S, Kaushik A, Gupta B (2016) Optimization of process parameters for controlled ring-opening polymerization of lactide to produce poly(L-lactide) diols as precursor for polyurethanes. Polym-Plast Technol Eng 55(17):1819–1830
Stridsberg KM, Ryner M, Albertsson A-C (2002) Controlled ring-opening polymerization: polymers with designed macromolecular architecture. Adv Polym Sci 157:41–65
Udoetok IA, Wilson LD, Headley JV (2018) Ultra-sonication assisted cross-linking of cellulose polymers. Ultrason Sonochem 42:567–576
Notley SM, Wågberg L (2005) Morphology of modified regenerated model cellulose II surfaces studied by atomic force microscopy: effect of carboxymethylation and heat treatment. Biomacromolecules 6:1586–1591
Gamelas JAF, Pedrosa J, Lourenço AF, Mutjé P, González I, Chinga-Carrasco G, Singh G, Ferreira PJT (2015) On the morphology of cellulose nanofibrils obtained by TEMPO-mediated oxidation and mechanical treatment. Micron 72:28–33
Aquino R, Veronese G, Carvalho AJF, Barbu E, Amaral AC, Trovatti E (2016) The potential of TEMPO-oxidized nanofibrillar cellulose beads for cell delivery applications. Cellulose 23:3399–3405
Peng Y, Gardner DJ, Han Y (2012) Drying cellulose nanofibrils: in search of a suitable method. Cellulose 19:91–102
Erbetta CAC, Alves RJ, Resende JM, Freitas RFS, Sousa RG (2012) Synthesis and characterization of poly(D,L-lactide-co-glycolide) copolymer. J Biomater Nanobiotechnol 3:208–225
Marais A, Kochumalayil JJ, Nilsson C, Fogelström L, Gamstedt EK (2012) Toward an alternative compatibilizer for PLA/cellulose composites: grafting of xyloglucan with PLA. Carbohydr Polym 89:1038–1043
Hatakeyama T, Nakamura K, Hatakeyama H (1982) Studies on heat capacity of cellulose and lignin by differential scanning calorimetry. Polymer 23:1801–1804
Gürdağ G, Sarmad S (2013) In: Kalia S, Sabaa MW (eds) Polysaccharide based graft copolymers. Springer, Berlin, p 15
Fukuzumi H, Saito T, Okita Y, Isogai A (2010) Thermal stabilization of TEMPO-oxidized cellulose. Polym Degrad Stab 95:1502–1508
Fukuzumi H, Saito T, Isogai A (2013) Influence of TEMPO-oxidized cellulose nanofibril length on film properties. Carbohydr Polym 93:172–177
Koval’aková M, Olčák D, Hronský V, Vrábel P, Fričová O, Chodák I, Alexy P, Sučik G (2016) Morphology and molecular mobility of plasticized polylactic acid studied using solid-state 13C- and 1H-NMR spectroscopy. J Appl Polym Sci 133:43517
Yeh J-T, Tsou C-H, Lu W, Li Y-M, Xiao HW, Huang CY, Chen K-N, Wu C-S, Chai W-L (2010) Compatible and tearing properties of poly(lactic acid)/poly(ethylene glutaric-co-terephthalate) copolyester blends. J Polym Sci B 48:913–920
Robles E, Salaberria AM, Herrera R, Fernandes SCM, Labidi J (2016) Self-bonded composite films based on cellulose nanofibers and chitin nanocrystals as antifungal materials. Carbohydr Polym 144:41–49
Tonoli GHD, Holtman KM, Glenn G, Fonseca AS, Wood D, Williams T, Sa VA, Torres L, Klamczynski A, Orts WJ (2016) Properties of cellulose micro/nanofibers obtained from eucalyptus pulp fiber treated with anaerobic digestate and high shear mixing. Cellulose 23:1239–1256
Barrau S, Vanmansart C, Moreau M, Addad A, Stoclet G, Lefebvre J-M, Seguela R (2011) Macromolecules 44:6496–6502
Li H, Huneault MA (2007) Effect of nucleation and plasticization on the crystallization of poly(lactic acid). Polymer 48:6855–6866
Srithep Y, Nealey P, Turng L-S (2013) Effects of annealing time and temperature on the crystallinity and heat resistance behavior of injection-molded poly(lactic acid). Polym Eng Sci 53:580–588
Choo K, Ching YC, Chuah CH, Julai S, Liou N-S (2016) Preparation and characterization of polyvinyl alcohol-chitosan composite films reinforced with cellulose nanofiber. Materials 9:644
Acknowledgements
This research was supported by a Research and Researchers for Industries (RRI) PhD Scholarship awarded by the Thailand Research Fund under the Office of the Prime Minister, Royal Thai Government (code: PHD57I0037), and PTT Global Chemical Public Company Limited, Thailand. We are grateful to Assistant Professor Dr Shingo Yokota for advice on FTIR measurements.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chuensangjun, C., Kanomata, K., Kitaoka, T. et al. Surface-Modified Cellulose Nanofibers-graft-poly(lactic acid)s Made by Ring-Opening Polymerization of l-Lactide. J Polym Environ 27, 847–861 (2019). https://doi.org/10.1007/s10924-019-01398-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-019-01398-y