Abstract
We describe a new macraucheniine macraucheniid, Micrauchenia saladensis gen. et sp. nov., from the late Miocene (Huayquerian SALMA). This is the first litoptern from Bahía Inglesa Formation, Chile. The specimen includes a partial mandible, cervical and thoracic vertebrae fragments, and portions of the forelimbs (a scapula fragment, an ulna-radius fragment, seven carpals, three metapodials, two proximal phalanges and four intermediate phalanges). The postcranial anatomy of Micrauchenia saladensis is consistent with terrestrial and cursorial locomotion, which suggests an allochthonous position of this specimen within the marine Bahía Inglesa Formation. The fusion of the ulna and radius and the presence of a radial aliform expansion align Micrauchenia with other macraucheniines, with which it shares these features. We interpret the fusion of the ulna and radius as a cursorial specialization and the aliform expansion as an adaptation for strong flexion movements and to resist higher transverse stresses during locomotion. In addition, Micrauchenia saladensis is the smallest member of the subfamily Macraucheniinae. To test the systematics and phylogenetics of this specimen, we expanded previous morphological matrices of macraucheniids by adding one dental and eight postcranial characters and scoring Micrauchenia saladensis. We performed maximum parsimony and Bayesian phylogenetic analyses, the latter applied for the first time to macraucheniid phylogeny. Our analyses confirm Micrauchenia saladensis as a member of the subfamily Macraucheniinae, although with uncertain affinities within this subfamily.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During most of the Cenozoic, South America was largely isolated from the rest of the continents, which allowed the independent evolution of a very particular mammalian fauna of South American ungulates (SANUs), xenarthrans and metatherians (Simpson 1980). This isolation was sporadically interrupted by mammalian immigrants arriving through rafting events during the Paleogene, probably from Africa (Antoine et al. 2012; Bond et al. 2015; Seiffert et al. 2020). The almost complete isolation of this fauna was finally put to an end by the gradual appearance of a terrestrial connection between South America and North America, the Isthmus of Panama, that fully connected both continents by 2.8 Ma (O’Dea et al. 2016), permitting immigration pulses from North America to South America and vice versa, in an event known as the Great American Biotic Interchange (GABI).
SANUs traditionally comprise five orders, Xenungulata, Notoungulata, Litopterna, Pyrotheria and Astrapotheria, with uncertain phylogenetic affinities. These orders were grouped in the mirorder Meridiungulata (McKenna 1975) probably due to biogeographic reasons and without any anatomical justification (Cifelli 1993). However, recent molecular studies have provided support for a close affinity between litopterns and notoungulates, which were both found to be closely related to perissodactyls (Buckley 2015; Welker et al. 2015; Westbury et al. 2017).
Among SANUs, litopterns were the most diverse order after notoungulates with 67 genera and nine families (Pascual et al. 1996; Croft et al. 2020) currently recognized, including species ranging from the base of the middle Paleocene (63.8 to 63.2 Ma, Peligran South American Land Mammal Age [SALMA]; Bonaparte and Morales 1997; Woodburne et al. 2014a, b) to the late Pleistocene/early Holocene (Tonni 1990; Bond 1999; Prado et al. 2015) in South America, and the Eocene in West Antarctica (Bond et al. 2006; Gelfo et al. 2017). Anatomically, litopterns tend to be the most similar SANUs to extant ungulates in terms of dental, cranial and postcranial proportions, and also present cursorial postcranial adaptations early in their evolutionary history (Scott 1910; Croft et al. 2020). Although most Paleogene taxa (e.g., Protolipternidae, Notonychopidae, Sparnotheriodontidae, Indaleciidae) have uncertain affinities (Cifelli 1993; Rose 2006; Croft et al. 2020), Neogene taxa are classified into three well-defined families: Macraucheniidae, Protherotheriidae, and Adianthidae (Cifelli 1983a; Soria 2001; Gelfo et al. 2016). Of these families, macraucheniids were small to large-sized litopterns (i.e., 53—1200 kg) with long necks, three-toed feet, and a reduced nasal region, with a trend towards retraction of the nasals (Bond 1999; Vizcaíno et al. 2012).
Macraucheniids are distinguished from adianthids mostly based on dental features and size, as not much is known about the adianthid postcranium. For instance, adianthids present a trilobed m3 and fossettes in P3-M3 formed by hypertrophied conular cristae (Cifelli and Soria 1983; Cifelli 1993). Proterotheriids differ from macraucheniids in several dental features, such as the hypocone and protocone in the upper molars connected by a crest (Cifelli 1993) and postcranial features like a reduction (or loss) of lateral digits II and IV and an enlarged digit III, similar to modern horses. Considering previous taxonomic propositions (Cifelli 1983a; Soria 2001; Gelfo et al. 2016), the first occurrence for Macraucheniidae would be Polymorphis (Roth 1899) from the Mustersan (late Eocene) of Patagonia, Argentina (Schmidt and Ferrero 2014), while the last occurrence is from the late Pleistocene/early Holocene, with the genera Macrauchenia (Bond 1999; Prado et al. 2015) and Xenorhinotherium (Cartelle and Lessa 1988). However, this age range excludes the poorly known Victorlemoinea and other sparnotheriodontids, the former previously considered within Macraucheniidae (Simpson 1945, 1948), which if proven correct, could imply an earlier origin for the family (early Eocene, Itaboraian SALMA).
Macraucheniidae is usually separated into two subfamilies: “Cramaucheniinae” (Eocene to middle Miocene) and Macraucheniinae (late Miocene to late Pleistocene/early Holocene), although only the latter has been shown to be monophyletic in phylogenetic analyses (Schmidt and Ferrero 2014; Forasiepi et al. 2016; McGrath et al. 2018). The most characteristic feature that distinguishes these two groups is that macraucheniines present a retracted nasal aperture to a more centrodorsal position, which suggests the presence of a proboscis (Burmeister 1864a; Scott 1910; Rusconi 1957; Bond 1999). Macraucheniines also have fused zeugopodial elements in the forelimbs (ulna-radius) and hindlimbs (tibia-fibula; Soria 1981).
Here we describe a new species, Micrauchenia saladensis gen. et sp. nov., that represents the first macraucheniid (and SANU) from the Tortonian-Messinian levels (Huayquerian SALMA) marine deposits of the Bahía Inglesa Formation (middle Miocene-late Pliocene), Chile, and also the first late Miocene macraucheniid from the west coast of South America (Fig. 1). In addition, we expanded previous character-taxon matrices of macraucheniids with the addition of novel dental and postcranial characters in order to test the position Micrauchenia within the family Macraucheniidae and also to better resolve the phylogenetic affinities of the different species of the family.
Geographic distribution of late Miocene sites yielding macraucheniids. 1. Quebrada Remolón, Atacama Region, Chile (Bahía Inglesa Formation, Huayquerian); 2. Acre region, southwestern Amazonia (Solimões Formation, “Mesopotamian” [Cozzuol 2006]); 3. Valle de Santa María, Catamarca, Argentina (“Estratos araucanos”, Chasicoan to Montehermosan [Rovereto 1914; Forasiepi et al. 2016]); 4. Conglomerado osífero, Entre Ríos, Argentina (Ituzaingó Formation, “Mesopotamian” [Rusconi 1932; Schmidt 2013; Forasiepi et al. 2016]); 5. Arroyo La Petra, San Luis, Argentina (Río Quinto Formation, “Mesopotamian” [Cerdeño et al. 2008]); 6. Huayquerías de San Carlos, Mendoza, Argentina (Huayquerías Formation, Huayquerian [Soria 1986; Forasiepi et al. 2016]); 7. Laguna Epecuén, and Laguna del Monte, Buenos Aires, Argentina (Cerro Azul Formation, Huayquerian [Soria 1986; Forasiepi et al. 2016; Schmidt et al. 2022]); 8. Salinas Grandes de Hidalgo, Telén, Guatraché, and Laguna Chillhué, La Pampa, Argentina (Cerro Azul Formation, Huayquerian [Schmidt et al. 2022]). 9. Arroyo Chasicó, Buenos Aires, Argentina (Arroyo Chasicó Formation, Chasicoan [Bond and López 1995; Schmidt and Ferrero 2014]). 10. Cantera Relleno Sanitario, Buenos Aires, Argentina (Cerro Azul Formation, Huayquerian [Schmidt et al. 2022]). The potentially late Miocene record of Scalabrinitherium ferreriai and ?Oxyodontherium zeballosi from “Barrancas de San Gregorio”, Uruguay (Kraglievich 1932; Perea et al. 2013), has been omitted, as it does not have a good stratigraphic control (i.e., it was found washed out on the beach). Each taxon is represented by a different colour. The silhouette used for the macraucheniid taxa was extracted from http://www.phylopic.org/ (by Zimices) and reproduced under a Creative Commons Attribution-ShareAlike 3.0 Unported (https://creativecommons.org/licenses/by-sa/3.0/)
Geographic and geologic context
The specimen SGO.PV.21700 was collected in 2005 in Quebrada Remolón, a creek with an outcrop of the Bahía Inglesa Formation, located in the Bahía Salado area, ~70 km south of Caldera, Atacama Region, Chile (Figs. 1 and 2a). SGO.PV.21700 was deposited in the Paleontology Area of the National Museum of Natural History of Santiago de Chile by unknown collectors, who left labels with information about the year and site of collection that allowed us to place it in a geological context. The Bahía Inglesa Formation has a complex shallow marine to coastal depositional history, representing a shoreline affected by eustatic changes, and tectonic subsidence and uplift, reflected in a diverse array of deep to shallow marine facies and sub environments (Rojo 1985; Marquardt et al. 2000; Le Roux et al. 2016). The formation was deposited in a forearc Neogene basin, overlying in nonconformity to metamorphic and crystalline basements, or in disconformity to the continental lower Miocene Gravas de Angostura Beds. In addition, it often underlies in disconformity to the Quaternary Estratos de Caldera Beds or to recent unconsolidated Holocene deposits (Godoy et al. 2003). It also has a coquina facies, which create a conspicuous white ridge at the top of the main outcrops.
Bahía Salado locality and stratigraphy. a. context map of the Bahía Salado locality, at the central coast of Atacama Region, Northern Chile. SGO.PV.21700 site at Quebrada Remolón and other relevant sites are highlighted. The main stratigraphic sections were made on the southwestern exposures. Geographic coordinates are in WGS84 datum. Basemap by ArcGIS 10.3 Geoeye/DigitalGlobe satellite composite of 2016; b. general stratigraphic section of Quebrada Hambre locality, with main contact relationships, topographic heights, and the correlative stratigraphic level of the SGO.PV.21700 remains, near the top of Bahía Inglesa Formation and below the local Phosphorite reference layer. The main facies are bioclastic conglomerates of coastal to closed shallow marine environments
The Bahía Inglesa Formation has been dated as middle Miocene to late Pliocene based on its fossil content (e.g., Rojo 1985; Suárez et al. 2004), Sr stable isotopic data on fossil invertebrates (Achurra 2004; Henriquez 2006), and K/Ar absolute radiometric data (Marquardt 1999; Godoy et al. 2003). The type locality of the Bahía Inglesa Formation at Los Dedos, Los Negros and surroundings has been interpreted as littoral deposits. Farther south, at the Chorrillos locality, deep marine fan and channels were inferred (Carreño 2012; Le Roux et al. 2016). Near the Salado Bay and in Quebrada Remolón, ~50 km south of Chorrillos area, the main lithofacies represented are big lenticular diatomite levels overlaid by coquinas and a metric reddish phosphorite layer, which is used as regional guide (Cuitiño et al. 2021; Fig. 2b). In a broad lithocorrelation, these lithofacies are equivalent to the Mina Fosforita Member present at the type locality, 7 m above an interbedded reworked crystalline tuff. K/Ar radiometric data of the tuff at the Mina Fosforita Member type section (sensu Le Roux et al. 2016), near the middle portion of the formation, indicates an age of 7.6 ± 1.3 Ma (Godoy et al. 2003), constraining this unit to the Tortonian-Messinian. As SGO.PV.21700 was found in Quebrada Remolón, within a unit equivalent to the Mina Fosforita Member, we estimate its age to be ~8–6 Ma.
Paleoenvironmental conditions of the Bahía Inglesa Formation
At the main classical phosphatic exposures of the Bahía Inglesa Formation, more than 60 species of mostly marine vertebrates have been found, including chondrichtyan fishes (rays, chimeroids, and sharks), sea birds (penguins, cormorants, boobies, petrels, and albatrosses), reptiles (crocodiles), and marine mammals (dolphins, whales, dugongs, seals and marine sloths; Le Roux et al. 2016). Findings of continental vertebrates in sediments of Bahía Inglesa Formation are rare. Remains of longirostrine crocodyliforms have been described in several localities of the unit (Walsh and Suárez 2005; Soto-Acuña et al. 2015). Today, the extant longirostrine crocodiles from the clade Gavialidae are restricted to freshwater environments (Grigg and Kirshner 2015). However, it is known that the Paleogene “thoracosaurs”, which are considered putative stem-gavialoids, inhabited marine environments (Hua and Jouve 2004; Delfino et al. 2005). The same is also true for the early diverging gavialid Sacacosuchus cordovai and the gryposuchine gavialid Piscogavialis jugaliperforatus, both from the Pisco Formation (Kraus 1998; Salas-Gismondi et al. 2022). Therefore, the crocodyliforms from Bahía Inglesa Formation are not necessarily reliable environmental indicators for terrestrial conditions. Nevertheless, a fragment of a capybara tooth conferred to Cardiatherium sp. was found in the Bahía Inglesa Formation, which constitutes the first non-marine vertebrate from this formation (Gutstein et al. 2007; Deschamps et al. 2013).
The complex lithofacies array of the succession suggest that the broad system was changing from closed shallow marine to littoral environments, in a relative short time span (Le Roux et al. 2016; Cuitiño et al. 2021). This condition allows the occurrence of typically continental vertebrates in coastal deposits. One of the best examples of shallow marine units bearing continental vertebrates in South America is the Camacho Formation (late Miocene) in Uruguay, which also contains a large assemblage of allochthonous Huayquerian vertebrate fossils in shallow marine to paralic environments (Perea and Martínez 2004; Perea et al. 2013; Soibelzon et al. 2019). Considering the similar general context, we suggest that the Micrauchenia saladensis remains are allochthonous in the clastic bearing levels, which is consistent with coquinas and very coarse sandstone to conglomerate layers bellow the phosphorite guide level. This depositional scenario occurred in a global eustatic high stage (Miller et al. 2005), but also with a local tectonic subsidence (Le Roux et al. 2016), both proper conditions for a significant variability in the former shoreline and the reworking of latter coastal to continental positions. Micrauchenia saladensis is the first record of Litopterna in this formation and the second record of a continental vertebrate in the Bahía Inglesa Formation.
Material and methods
Description and comparison
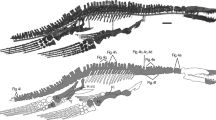
The anatomical descriptions were based on SGO.PV.21700, a specimen of a new macraucheniid from the Bahía Salado, Bahía Inglesa Formation, Chile. SGO.PV.21700 is deposited in the Paleontology Area of the National Museum of Natural History of Santiago de Chile. The specimen includes a mandible fragment with the right condylar and coronoid processes, an atlas fragment, an axis fragment, two cervical vertebrae fragments (fifth and seventh cervical vertebrae), one fragmentary thoracic vertebra (second thoracic vertebra), left scapula fragment, left ulna-radius fragment (mid-shaft to distal end preserved), seven carpal elements (partial left scaphoid, partial left and complete right lunate, left cuneiform, left pisiform, left magnus and right unciform), three metapodials (right Mc II, a fragmentary left Mc III and a fragmentary Mc II or Mc IV) and six phalanges (two proximal of the right digit II and left digit III, and four intermediate, one of the digit III and three of the digits II and/or IV; Fig. 3). The fossil bones were mechanically prepared in the Red Paleontológica U-Chile Laboratory of the University of Chile. The preparation was carried out with airscribes ME 9100 and MicroJacks under trinocular microscopes and stabilized by consolidants such as paraloid (B-72) and cyanoacrylate.
Skeletal reconstruction of Micrauchenia saladensis gen. et sp. nov. (SGO.PV.21700) in left lateral view, with preserved elements in red. The right portion of the mandible, right unciform, and left scaphoid have been mirrored in order to be visible in the figure. The second thoracic vertebra (T2) is preserved but is not visible in the figure as it is behind the left scapula. The anatomy of the missing elements is based in other macraucheniids as Theosodon (Scott 1910), Promacrauchenia (Soria 1986), Macrauchenia (PIMUZ A/V 5700; MACN PV 2) and Xenorhinotherium (MCL 2643/03). In the limbs, Hindu-Arabic numerals indicate the phalanx position from proximal to distal (1 to 3), and Roman numerals indicate the digit position (II to IV). Abbreviations: C5, fifth cervical vertebra; C7, seventh cervical vertebra; cu, cuneiform; lu, lunate; ma, magnum; Mc, metacarpal; pi, pisiform; sc, scaphoid; un, unciform. Scale bar equals 10 cm
Comparisons were made with fossil specimens described and photographed in the literature and/or from direct observation/images from museum collections of taxa that have been recognized by anatomical features and/or phylogenetic studies as members of the family Macraucheniidae and that also preserve postcranial remains (e.g., Scott 1910; Sefve 1925; Parodi 1931; Cartelle and Lessa 1988). In particular, we compared SGO.PV.21700 with Cramauchenia Ameghino, 1902 (late Oligocene to early Miocene, Deseadan to Colhuehuapian SALMAs), Coniopternium Ameghino, 1894a (late Oligocene, Deseadan SALMA), Theosodon Ameghino, 1887 (early Miocene to late middle Miocene, Colhuehuapian-Laventan SALMAs), Scalabrinitherium Ameghino, 1883 (Kraglievich 1932; late Miocene to early Pliocene, Huayquerian to Montehermosan SALMAs), Cullinia Cabrera and Kraglievich, 1931 (late Miocene, Chasicoan to Huayquerian SALMAs), Promacrauchenia Ameghino, 1904 (late Miocene to early Pleistocene, Huayquerian to Marplatan SALMAs), Macrauchenia Owen, 1838 (late Pliocene, Marplatan SALMA to late Pleistocene/early Holocene) and Xenorhinotherium Cartelle and Lessa, 1988 (late Pleistocene/early Holocene). Most measurements were taken either manually using digital callipers, or digitally using Fiji (ImageJ v2.1.0; Schindelin et al. 2012), to the nearest two decimal places. Some measurements were taken directly from the literature for comparisons. A complete list of the comparison material, institutional numbers and measurements is given in Online Resource 1 (Tables S1, S2, S3, S4, S5, S6 and S7).
As the subfamily Cramaucheniinae has previously been shown to be paraphyletic (Schmidt and Ferrero 2014; Forasiepi et al. 2016; McGrath et al. 2018), it cannot be considered a natural group. As such we used the term “cramaucheniines” throughout the article to talk about early macraucheniids that are outside the monophyletic subfamily Macraucheniinae.
We followed the Nomina Anatomica Veterinaria (International Committee on Veterinary Gross Anatomical Nomenclature 2017) for the anatomical terminology in general, and for carpal terminology, we followed Rose (2006). When we made soft tissue inferences in anatomical descriptions, we mostly followed veterinary anatomy books (Sisson 1914; Evans and Lahunta 2012; Constantinescu et al. 2018; Denoix 2019; Aurich et al. 2020), which contain anatomical information on some modern laurasiatheres, including perissodactyls, the closest living relatives of litopterns according to molecular evidence (Buckley 2015; Welker et al. 2015; Westbury et al. 2017). In some cases, we used additional references, which we cite accordingly in the text.
Character dataset and taxa included
To assess the phylogenetic affinities of SGO.PV.21700, we compiled a data matrix of 43 characters scored for 21 taxa. This dataset is modified from previous matrices (Schmidt and Ferrero 2014; Forasiepi et al. 2016) with the addition of a new craniodental character and eigth postcranial characters (Online Resource 2). The matrix is available online on Morphobank (morphobank.org) under the project number 3933 and as Nexus files (Online Resources 3 and 4). Even though the likely ancestral dental formula for litopterns is I1/i1 I2/i2 I3/i3 P1/p1 P2/p2 P4/p4 P5/p5 M1/m1 M2/m2 M3/m3 considering the probable loss of P3/p3 in the common ancestor of Placentalia (McKenna 1975; Novacek 1986; O’Leary et al. 2013), we kept the dental formula used by previous authors for macraucheniids of I1/i1 I2/i2 I3/i3 P1/p1 P2/p2 P3/p3 P4/p4 M1/m1 M2/m2 M3/m3 (e.g., Forasiepi et al. 2016) as it is more intuitive and does not have a negative impact in dental comparisons between litopterns (i.e., the comparisons are still homologous). We included the same taxa as Forasiepi et al. (2016) with the addition of Llullataruca shockeyi (McGrath et al., 2018). Additionally, we considered the small differences between Huayqueriana cf. H. cristata (Forasiepi et al. 2016) and Huayqueriana cristata Rovereto, 1914, as intraspecific variation of the latter taxon. Therefore, it was scored as a single taxon in the matrix, treating any contradictory scores as polymorphic. We chose Tricoelodus Ameghino, 1897 as the outgroup because is one the best-known and most complete adianthids from the late Oligocene (Deseadan SALMA) and has been used in previous analyses (Schmidt and Ferrero 2014; Forasiepi et al. 2016; McGrath et al. 2018). However, in contrast to latter studies, we removed the adianthid Proadiantus excavatus Ameghino, 1897 because is poorly known, having considerably fewer scored characters than Tricoelodus (11 vs 16). For the 34 craniodental characters of previous matrices (Schmidt and Ferrero 2014; Forasiepi et al. 2016), we used the character scores from McGrath et al. (2018), scoring additionally character 15 in Polymorphis lechei as it was possible to assess examining the holotype (MLP 12–2168). The nine new characters were scored based on direct observation of museum specimens, pictures of the specimens, and images/descriptions from the literature. Finally, we scored the Bahía Salado specimen (SGO.PV.21700) and also the dental and postcranial Promacrauchenia sp. material from Inchasi (Anaya and MacFadden 1995) into the matrix. The latter was included because its peculiar anatomy and the fact that its taxonomic assignment has been previously questioned (Schmidt 2013). Characters 1, 2, 3, 7 and 8 were treated as ordered, as they are part of a transformational sequence.
Phylogenetic analysis
Parsimony analysis We use TNT v1.5 (Goloboff and Catalano 2016), setting the outgroup as Tricoelodus spp. and employing a "New Technology" driven search using sectorial search, ratchet, drift and tree fusing, set to find the minimum length tree (best score) 100 times. This was followed by an additional “traditional search” using tree bisection and reconnection (TBR) branch swapping algorithm. As a result, we obtained 36 most parsimonious trees (MPTs) of 89 steps with consistency index (CI) = 0.607 and retention index (RI) = 0.701. The MPTs were then used to compute a strict consensus tree and a 50% majority rule tree. The absolute Bremer supports and Jackknife resampling (1000 replicates, 36%-character removal) for the nodes were computed for the strict consensus tree. During these analyses, SGO.PV.21700 was identified as a wildcard taxon probably due to the lack of scored craniodental characters, so it was removed a posteriori to analyse the topology of the strict consensus when this taxon is not present. As the topology excluding SGO.PV.21700 from the strict consensus tree was very similar to the 50% majority rule tree that included this taxon, we mapped apomorphies on the 50% majority rule tree.
Undated Bayesian analysis The undated Bayesian analysis was performed in MrBayes v3.2.7a (Ronquist et al. 2012) setting the outgroup as Tricoelodus spp (Online Resource 3). We used two partitions for analysing the morphological data: the Mkv + Γ model of morphological evolution (Lewis 2001) with four rate categories (Harrison and Larsson 2015) for the characters with three or more states, and the MkA + Γ model for the binary characters with four rate categories in order to consider asymmetrical forward and backward rates between character states (Pyron 2017). The analyses were run using two runs of four chains and 15 million Markov chain Monte Carlo (MCMC) generations, sampling every 1000 generations and discarding the 25% of the samples as burn-in. We ensured that the deviation of split frequencies was below 0.01 and that the effective sample size for the parameters was > 200.
Tip-dated Bayesian analysis The tip-dated Bayesian analysis was also performed in MrBayes v3.2.7a (Ronquist et al. 2012) with the same model of morphological evolution and sampling settings as the undated Bayesian analysis (see above; Online Resource 4). The root of the tree was calibrated using an exponential distribution with a minimum age of 42 Ma and a mean of 44 Ma. The minimum age for the root was based in the maximum age of the most basal putative adianthid Proectocion (Barrancan SALMA, 42–39 Ma; Woodburne et al. 2014a).
Ideally, when defining calibration bounds for each taxon, these should be based on the radiometric age uncertainty associated to the specific specimens scored in the morphological matrix (Püschel et al. 2020). However, the institutional numbers of the specimens used in the original matrices and their stratigraphic provenience has not been reported (Schmidt and Ferrero 2014; Forasiepi et al. 2016), and this information is not currently available (personal communication, G. Schmidt, 2021). Therefore, the calibration bounds were based on the presence data of Croft et al. (2020) for each SALMA, and the radiometric information currently available for defining each time interval (Table S7 in Online Resource 2). We used a uniform distribution between the maximum and minimum age associated with each taxon to represent the radiometric age uncertainties with one exception. As we only considered the Santacrucian specimens of Theosodon for character scoring, the age interval for the tip-dating Bayesian analysis for Theosodon spp. was the Santacrucian instead of the whole time interval of all the specimens referred to this genus (i.e., Colhuehuapian to Laventan [21.0–11.8 Ma]). There is undoubtedly a need to revise the taxonomy of this genus, as previous authors have recognized (Cifelli and Guerrero Diaz 1997; Croft et al. 2004; Schmidt and Ferrero 2014; McGrath et al. 2018), with part of the problem being the number of species based on fragmentary remains from different time intervals, which generates a disagreement in the exact number of valid species between different authors (e.g., ten [Croft et al. 2004], seven [Cassini et al. 2012], eight [McGrath et al. 2020]). However, a revision of this nature is beyond the scope of this work.
We used a gamma distributed clock rate prior, with a mean of 0.02462008 and standard deviation of 0.01095129. These priors were derived following the methodology of (Gunnell et al. 2018) using the previously obtained undated Bayesian consensus tree and the packages ape (Paradis et al. 2004) and fitdistrplus (Delignette-Muller and Dutang 2004) from R (R Core Team 2019). We selected the best model according to the Bayesian information criterion. Branch rate variation was modelled using the Independent Gamma Rate (IGR) relaxed clock model with an exponential distribution of rate 10 (default MrBayes setting). The FBD model was used as the prior on divergence times, using an exponential net diversification prior with rate 1, a beta turnover prior with shape parameters α = 1 and β = 1, a beta fossil sampling proportion prior with shape parameters α = 1 and β = 1 and an extant sampling proportion of 1. We use diffuse priors for the clock rate variance and the FBD model, which reflect the uncertainty in our prior expectation of how these parameters are distributed.
The analyses were run using two runs of four chains and 45 million Markov chain Monte Carlo (MCMC) generations, sampling every 1000 generations and discarding the 25% of the samples as burn-in. We ensured that the deviation of split frequencies was below 0.01 and that the effective sample size for the parameters was > 200.
Institutional abbreviations
ACM, Pratt Museum, Amherst College, Amherst, USA; AMNH, American Museum of Natural History, New York, USA; CICYTTP PV, Centro de Investigaciones Científicas y Transferencia de Tecnología a la Producción, Diamante, Entre Ríos, Argentina; IMMH, Idaho Museum of Natural History, Pocatello, Idaho; MUHNCAL-KM, Museo de Historia Natural y Cultural del Desierto de Atacama, Calama, Chile; MACN PV, Museo Argentino Ciencias Naturales “Bernardino Rivadavia”, Colección Nacional de Paleontología Vertebrados, Buenos Aires, Argentina; MAS PALEO- VERT, Museo de Ciencias Naturales y Antropológicas “Profesor Antonio Serrano” de Paraná, Entre Ríos; MCL, Museu de Ciências Naturais da Pontificia Universidade Católica de Minas Gerais, Paleontological collection, Belo Horizonte, Brazil; MLP, Museo de La Plata, La Plata, Argentina; MNHN-Bol, Departamento de Paleontología, Museo Nacional de Historia Natural, La Paz, Bolivia; MUSM, Departamento de Paleontología de Vertebrados, Museo de Historia Natural, Universidad Mayor de San Marcos, Lima, Perú; MNHN.F, Museum national d’Histoire naturelle, Palaeontological collection, Paris, France; MPCN, Museo Paleontológico y de Ciencias Naturales “Alejandro C. Berro”, Mercedes, Soriano, Uruguay; MPEF- PV, Colección del Paleontología del Museo Paleontológico “Egidio Feruglio”, Trelew, Argentina; NMS, National Museum of Scotland; PIMUZ, Paleontological Institute and Museum, University of Zurich; SGO.PV, Vertebrate Paleontology Collection, Museo Nacional de Historia Natural, Santiago, Chile; UATF-V, Vertebrate Paleontology Collections, Universidad Autónoma Tomás Frías, Potosí, Bolivia; YPM VPPU, Yale Peabody Museum, Vertebrate Paleontology Princeton University Collection, Yale, USA.
Systematic palaeontology
Mammalia Linnaeus, 1758
Eutheria Huxley, 1880
Panperissodactyla Welker et al., 2015
Litopterna Ameghino, 1889
Macraucheniidae Gervais, 1855
Macraucheniinae Ameghino, 1902
Emended diagnosis (modified from Soria 1981 and Schmidt 2013) small to large-sized macraucheniids (~53 to 1200 kg). Elongated rostrum. Nostrils in a dorsal position, close to the level of the orbits and posterior to dorsally projected maxillae. Nasals vestigial or absent. No sagittal crest. The coronal plane passing through infraorbital foramen is located in a position that varies between the anterior margin M2 and over M3. The left and right premaxillae and maxillae are sutured sagitally in dorsal view in contrast to earlier macraucheniids where the bones are separate. Palate narrows anteriorly at P2 or P3 level. Mesodont and selenodont molars. I1/i1-P1/p1 generally imbricated. P3-M3 with enamel fossettes in the trigon basin. Fused ulna and radius, and tendency towards the development of an aliform expansion on the radius. Tibia and fibula partially fused. Massive and less gracile calcaneus in comparison with earlier macraucheniids. Tridactyl manus and pes.
Micrauchenia, gen. nov.
LSID urn:lsid:zoobank.org:act:30B931AC-ED24-459D-A28A-DAE22EB7B9C1.
Figures 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 and 19
Mandible of Micrauchenia saladensis (SGO.PV.21700). This corresponds to a fragment of the right dorsoposterior portion of the element. a. lateral view; b. annotated line drawing in lateral view. Abbreviations: con proc, condylar process; cor cr, coronoid crest; cor proc, coronoid process; mas fos, masseteric fossa. Scale bar equals 2 cm
Atlas of Micrauchenia saladensis compared with atlases of other macraucheniids. a-f. atlas of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), dorsal (c), left lateral (d), ventral (e), and ventral (f; right fragment alone) views. g. atlas of a subadult Cramauchenia normalis (MLP 83-III-2–1) in ventral view. h. atlas of Theosodon lydekkeri (MACN A 9255) in ventral view. i. atlas of an early Pliocene macraucheniine indet. from Quequén Salado River (MACN PV 9779), possibly a subadult, in ventral view. The red arrows in g and h indicate the notch that separates the articular facet for the axis and the transverse process (absent in c-d and i). Abbreviations: lat v for, lateral vertebral foramen; occip, occipital; tr for, transverse foramen; tr proc, transverse process; vent tub, ventral tubercle; vert for, vertebral foramen. Scale bars equal 2 cm
Fifth and seventh cervical vertebrae (C5 and C7) of Micrauchenia saladensis (SGO.PV.21700). a-f. C5 in anterior (a), posterior (b), dorsal (c), left lateral (d), ventral (e), and posterolateral (f) views. g-l. C7 in anterior (g), posterior (h), dorsal (i), left lateral (j), ventral (k), and right lateral (l) views. Abbreviations: pos fov, posterior costal fovea; pos proc, posterior projection of the transverse process; postzyg, postzygapophysis; spin proc, spinous process; tr for, transverse foramen; tr proc, transverse process; tub, small anterior transverse process tubercle; vent keel, ventral keel; vert for, vertebral foramen. Scale bars equal 2 cm
Second thoracic vertebra (T2) of Micrauchenia saladensis (SGO.PV.21700). a. anterior view; b. posterior view; c. dorsal view; d. ventral view; e. left lateral view; f. right lateral view. Abbreviations: ant fov, anterior costal fovea; fov tran, costal fovea of transverse process; nu can, nutrient canal; prezyg, prezygapophysis; pos fov, posterior costal fovea; spin proc, spinous process; tr for, transverse foramen; tr proc, transverse process; tub, small posterior transverse process tubercle; vent keel, ventral keel; vert for, vertebral foramen. Scale bar equals 2 cm
Left scapula, and articulated ulna-radius and manus of Micrauchenia saladensis (SGO.PV.21700). a. scapula in lateral view. b-c. ulna-radius and manus articulated in anterior (b), and posterior (c) views. d-e. ulna-radius in medial (d), and lateral (e) views. The right digit II and the unciform were mirrored in b-c. Bones and facets are indicated in bold and regular font respectively. Abbreviations: ae, aliform expansion of the radius; cu, cuneiform; fos inf, fossa infraspinata; fos sup, fossa supraspinata; lu, lunate; ma, magnum; pi, pisiform; ra, radius; sc, scaphoid; sca spi, scapular spine; tr, trapezium; trd, trapezoid; ul, ulna; un, unciform. Scale bar equals 4 cm in a and d, and 5 cm in b-c
Left ulna-radius and articulated carpals of Micrauchenia saladensis (SGO.PV.21700). a. left ulna-radius in distal view. b-c. left articulated carpals in proximal (a), and distal (c) views. Bones and facets are indicated in black bold and black regular font respectively. Abbreviations: cu, cuneiform; lu, lunate; ma, magnum; Mc, metacarpal; pi, pisiform; ral, radius lateral facet; ram, radius medial facet; sc, scaphoid; tr, trapezium; trd, trapezoid; ula, ulnar anterior facet; ulp, ulnar posterior facet; un, unciform. Scale bar equals 1 cm
Left scaphoid of Micrauchenia saladensis compared with scaphoids of other macraucheniids. a-f. scaphoid of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), proximal (c), distal (d), medial (e), and lateral (f) views. g-h. scaphoid of Theosodon sp. (MLP 12–883) in distal (g), and lateral (h) views. i-j. scaphoid of a Pliocene Macraucheniinae indet. (MLP 57-X-10–157) in distal (i), and lateral (j) views. k-l, scaphoid of Macrauchenia patachonica in distal (k), and lateral (l) views. The red arrow indicates the ridge separating the anterior and posterior trapezoid facets. The scaphoids in g-j were mirrored to facilize comparisons. Abbreviations: ant, anterior; lat, lateral; lud, lunate distal facet; lup, lunate proximal facet; ma, magnum facet; med, medial; post, posterior; prox, proximal; ram, radius medial facet; tr, trapezium facet; trd, trapezoid facet; trda, trapezoid anterior facet; trdp, trapezoid posterior facet. Scale bar equals 1 cm in a-j, and 2 cm in k-l
Right lunate of Micrauchenia saladensis compared with lunates of other macraucheniids. a-f. lunate of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), proximal (c), distal (d), medial (e), and lateral (f) views. g-h. lunate of Coniopternium primitivum (MNHN.F.SAL1051) in anterior (g), and medial (h) views. i-j. lunate of Promacrauchenia antiquua (MACN PV 7986) in anterior (i), and medial (j) views. k-l. lunate of Macrauchenia patachonica (MNHN.F.PAM75) in anterior (k), and medial (l) views. The lunates in g-h and k-l were mirrored to facilize comparisons. Abbreviations: ant, anterior; cu, cuneiform facet; lat, lateral; ma, magnum facet; med, medial; prox, proximal; ral, radius lateral facet; scd, scaphoid distal facet; scp, scaphoid proximal facet. Scale bar equals 1 cm in a-j, and 2 cm in k-l
Left cuneiform of Micrauchenia saladensis compared with cuneiforms of other macraucheniids. a-f. cuneiform of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), proximal (c), distal (d), medial (e), and lateral (f) views. g-h. cuneiform of Theosodon sp. (MLP 12–877) in proximal (g), and distal (h) views. i-j. cuneiform of Promacrauchenia antiquua (MACN PV 7986) in proximal (i), and distal (j) views. k-l. cuneiform of Macrauchenia patachonica (MNHN.F.PAM75) in proximal (k), and distal (l) views. The cuneiform in g-h was mirrored to facilize comparisons. Black dashed lines indicate the limit between two facets. The red arrow indicates an anteroposterior elongate ridge dividing the pisiform facet in Theosodon sp.. Abbreviations: ant, anterior; lat, lateral; lu, lunate facet; ma, magnum facet; med, medial; pi, pisiform facet; post, posterior; prox, proximal; ula, ulnar anterior facet; un, unciform facet. Scale bar equals 1 cm in a-j, and 2 cm in k-l
Left pisiform of Micrauchenia saladensis compared with pisiforms of other macraucheniids. a-f. pisiform of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), proximal (c), distal (d), medial (e), and lateral (f) views. g-h. pisiform of Cullinia levis? (MLP 29-IX-1–78; holotype) in anterior (g), and medial (h) views. i-j. pisiform of Cullinia cf C. levis (MLP 55-IV-28–97) in anterior (i), and medial (j) views. k-l. pisiform of Promacrauchenia antiquua (MACN PV 7986) in anterior (k), and medial (l) views. m–n. pisiform of Macrauchenia patachonica (MNHN.F.PAM75) in anterior (m), and medial (n) views. The red arrow is pointing to the distal swelling of the pisiform of Cullinia levis? (MLP 29-IX-1–78; holotype), not seen in other macraucheniines. The pisiforms in g-l were mirrored to facilize comparisons. Abbreviations: ant, anterior; cu, cuneiform facet; lat, lateral; med, medial; post, posterior; prox, proximal; ulp, ulnar posterior facet. Scale bar equals 1 cm in a-l, and 2 cm in m–n
Left magnum of Micrauchenia saladensis compared with magnums of other macraucheniids. a-f. magnum of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), proximal (c), distal (d), medial (e), and lateral (f) views. g-h. magnum of Coniopternium primitivum (MNHN.F.SAL1050) in anterior (g), and proximal (h) views. i-j. magnum of Theosodon gracilis (MACN A 2569–2608) in anterior (i), and proximal (j) views. k-l. magnum of Macrauchenia patachonica (MNHN.F.PAM75) in anterior (k), and proximal (l) views. Black dashed lines indicate the limit between two facets. The magnum in i-j were mirrored to facilize comparisons. Abbreviations: ant, anterior; cu, cuneiform facet; lat, lateral; lu, lunate facet; Mc, metacarpal; med, medial; post, posterior; prox, proximal; sc, scaphoid facet; trd, trapezoid facet; un, unciform facet. Scale bar equals 1 cm in a-j, and 2 cm in k-l
Right unciform of Micrauchenia saladensis compared with unciforms of other macraucheniids. a-f. unciform of Micrauchenia saladensis (SGO.PV.21700) in anterior (a), posterior (b), proximal (c), distal (d), medial (e), and lateral (f) views. g-h. unciform of Cramauchenia normalis (MNHN.F.COL183) in proximal (g), and medial (h) views. i-j. unciform of Theosodon gracilis (MACN A 2569–2608) in proximal (i), and medial (j) views. k-l. unciform of Macrauchenia patachonica (MNHN.F.PAM75) in proximal (k), and medial (l) views. The unciform in i-l were mirrored to facilize comparisons. Abbreviations: ant, anterior; cu, cuneiform facet; lat, lateral; ma, magnum facet; Mc, metacarpal; med, medial; post, posterior; prox, proximal. Scale bar equals 1 cm in a-j, and 2 cm in k-l
Metacarpals of Micrauchenia saladensis (SGO.PV.21700) and Macrauchenia patachonica (MNHN.F.PAM75). a-b, e–f. right Mc II of Micrauchenia saladensis in medial (a), lateral (b), proximal (e), and distal (f) views. c-d, g-h. left Mc III of Micrauchenia saladensis in medial (c), lateral (d), proximal (g), and distal (h) views. i. Mc II or Mc IV? of Micrauchenia saladensis in distal view. j-k, n–o. left Mc II of Macrauchenia patachonica (mirrored) in medial (j), lateral (k), proximal (n), and distal (o) views. l-m, p-q. left Mc III of Macrauchenia patachonica in medial (l), lateral (m), proximal (p), and distal (q) views. Abbreviations: a, anterior facet; coll lig, insertion for metacarpophalangeal collateral ligaments; lat, lateral; ma, magnum facet; Mc, metacarpal; med, medial; p, posterior facet; tr, trapezium facet; trd, trapezoid facet; un, unciform facet. Scale bar equals 1 cm in a-i, and 2 cm in j-q
Proximal phalanges of the manus of Micrauchenia saladensis (SGO.PV.21700). a-d. right proximal phalanx of digit II in medial (a), lateral (b), proximal (c), and distal (d) views; e–h. left proximal phalanx of digit III in medial (e), lateral (f), proximal (g), and distal (h) views. i. left proximal phalanx of digit II of Macrauchenia patachonica (MNHN.F.PAM75; mirrored) in proximal view. j. left proximal phalanx of digit III of Macrauchenia patachonica (MNHN.F.PAM75; mirrored) in proximal view. Abbreviation: coll lig, insertion for interphalangeal collateral ligament. Scale bar equals 1 cm in a-i, and 2 cm in j-k
Intermediate phalanges of Micrauchenia saladensis (SGO.PV.21700). a-e. right intermediate phalanx of digit II in dorsal (a), medial (b), lateral (c), proximal (d), and distal (e) views. f. left intermediate phalanx of digit IV in dorsal view. g. left intermediate phalanx of digit II or right intermediate phalanx of digit IV in dorsal view. h–l. left intermediate phalanx of digit III in dorsal (h), medial (i), lateral (j), proximal (k), and distal (l) views. Only the most demarcated depressions for the interphalangeal collateral ligament are pointed. Abbreviation: coll lig, insertion for interphalangeal collateral ligament. Scale bar equals 1 cm
Type species Micrauchenia saladensis.
Diagnosis Small (53–103 kg) macraucheniid, similar in size to Cramauchenia normalis and Coniopternium andinum and smaller than Cullinia levis, previously the smallest known macraucheniine. The posteriormost border of the transverse processes of the atlas are posterior to the articular facets for the axis as in Macrauchenia but in contrast to Theosodon where the transverse processes are anterior to the articular facets for the axis. The articular facets for the atlas of the axis are dorsoventrally low in relation to its mediolateral width (WA/HA > 0.9) more similar to Macrauchenia than to Theosodon, in which these facets are markedly relatively higher. The ulna and radius are completely fused, and the radius presents a well-developed medial aliform expansion or flange similar to Promacrauchenia in its degree of expansion. However, the ulna-radius is wider mediolaterally at this point than the width of this element measured at the level of the distal articular surfaces (URA/URD > 1) similar to Macrauchenia but in contrast to what is seen in Promacrauchenia where URA/URD < 1. The cuneiform and unciform extend over the magnum proximally as in Macrauchenia but in contrast to Theosodon, where the cuneiform-magnum and unciform-magnum articulations are entirely lateral. The pisiform is greatly proximodistally expanded at the posterior end as in Cullinia, Promacrauchenia, Macrauchenia and Xenorhinotherium. The robust metapodials are proportionally similar in terms of relative length and width to Theosodon, in contrast to Cullinia levis, in which they are proportionally more slender and elongated.
Age and distribution Tortonian-Messinian, 8–6 Ma, Mina Fosforita Member, Bahía Inglesa Formation, Chile, temporally within the Huayquerian SALMA.
Etymology From the words “mikrós” and “aukhḗn”, meaning “small” and “neck” respectively in old Greek. The name refers to the small size of this macraucheniine in comparison to the Pleistocene genus Macrauchenia.
Micrauchenia saladensis, sp. nov.
LSID urn:lsid:zoobank.org:act:1FB315E5-1908-444B-897A-E7C313EA3C6B.
Holotype The specimen SGO.PV.21700 includes a right mandible fragment with the right condylar and coronoid processes, an atlas fragment, an axis fragment, two cervical vertebrae fragments (fifth and seventh cervical vertebrae), one fragmentary thoracic vertebra (second thoracic vertebra), left scapula fragment, left ulna-radius fragment (mid-shaft to distal end preserved), seven carpal elements (partial left scaphoid, partial left and complete right lunate, left cuneiform, left pisiform, left magnus and right unciform), three metacarpals (right Mc II, a fragmentary left Mc III and a fragmentary Mc II or Mc IV) and six phalanges (two proximal of the right digit II and left digit III, and four intermediate, one of the digit III and three of the digits II and/or IV; Fig. 3).
Etymology From Bahía Salado, the locality in which the type and only specimen was found.
Type locality Quebrada Remolón, Bahía Salado, Copiapó Province, Atacama Region, Northern Chile.
Age and distribution As for genus.
Diagnosis As for genus.
Descriptions and comparisons
Skull
Mandible The right mandible fragment preserves just the dorsal portion of the ramus with the coronoid and the condylar processes (Fig. 4 and Table S1 ion Online Resource 1). The condylar process is small, cylindrical and posteriorly has a slightly convex articular facet where it articulates with the glenoid fossa (fossa mandibularis) on the squamosal. The mandibular notch (incisura mandibulae) is anteroposteriorly very narrow. The coronoid process is anteroposteriorly narrow, dorsoventrally elongated and mediolaterally slender. The apex of the coronoid process is tapered posteriorly and terminates posterior to the condylar process. Anteriorly, it presents a distinct coronoid crest that is very strong dorsally but as it continues ventrally it becomes more subtle. Laterally, the coronoid process is slightly concave near its tip for the attachment of the temporalis muscle (Evans and Lahunta 2012; Constantinescu et al. 2018). On the ramus portion, preserved below the level of the condylar process, there is a concavity laterally, which is probably part of the attachment area for the masseter (Evans and Lahunta 2012; Constantinescu et al. 2018). There is no sign of a condyloid crest in contrast to Cramauchenia normalis (MNHN.F.COL181) and Macrauchenia (MACN PV 2), which present a subtle but noticeable condyloid crest dividing the masseteric fossa into two halves. In Theosodon, the condyloid crest may be variable, as there appears to be a very subtle ridge in Theosodon garretorum (YPM VPPU 15164; Scott 1910: pl. XVII, fig. 1) but this is absent in Theosodon lydekkeri specimens (MACN A 2490; MACN A 9269–88). Llullataruca shockeyi (UATF-V-001904) also seems to lack a condyloid crest (McGrath et al. 2018). Apart from differences in the development of the condyloid crest and differences in size, the general outline of this element is very similar to Miocene macraucheniids like Theosodon (YPM VPPU 15164) and Llullataruca (UATF-V-001904), and also to more derived Pleistocene macraucheniids like Macrauchenia (MACN PV 2), which indicates that over time this element has been mostly conserved within this family.
Postcranial elements
Vertebrae
The preserved elements of the vertebral column include the atlas, axis, two cervical vertebrae (fifth and seventh cervical vertebrae) and a thoracic vertebra (second thoracic vertebra) with different degrees of preservation (Figs. 5-8). In terms of absolute size, the vertebrae of Micrauchenia saladensis are on average of similar size than those of Cramauchenia normalis, ~27% smaller than Theosodon spp., and 2–3 times smaller than Macrauchenia patachonica and Xenorhinotherium bahiense (Table S2 in Online Resource 1).
Atlas The atlas is broken, preserving almost the entire left half and only part of the right half in fragments that are separated by a fracture in the dorsal arch, however, it is possible to infer its whole anatomy (Fig. 5a-f). Although part of the dorsal arch is missing, there is no indication of a marked dorsal tubercle (tuberculum dorsale), with the arch being moderately high but rounded. Positioned anteriorly are wide and mostly concave articular facets for the occipital condyles of the cranium or occipital facets which are closer ventrally to each other than dorsally, being convex in a small area at their ventromedial portion (Fig. 5a). Posteriorly, the atlas of Micrauchenia saladensis presents almost flat oval articular facets for the axis and in the ventral part of the neural arch there is a concave facet for the odontoid process of the axis (Fig. 5b). Just ventral to the facet for the odontoid process, on the ventral aspect of the atlas, there is a small but distinct ventral tubercle (tuberculum ventrale) that extends the length of the atlas becoming less prominent anteriorly (Fig. 5d, e).
The transverse processes of the atlas or wings are moderately mediolaterally broad and anteroposteriorly elongated, ending anteriorly in an alar notch (incisura alaris) and posteriorly surpassing the articular facets for the axis terminating in a posteriorly projecting rounded lobe (Fig. 5c-e). Ventrally the transverse processes are moderately concave, and there is no atlantic fossa (fossa atlantis; Fig. 5d, e).
The alar notch of the atlas of M. saladensis is anteroposteriorly deep and mediolaterally narrow. The alar notch is similar to the same structure in the perissodactylamorph Cambaytherium (Rose et al. 2019), the perissodactyl Hyracotherium (Wood et al. 2011) and to Canis and other carnivorans (Evans and Lahunta 2012); in the case of the latter, it has been observed to be a point of passage of the vertebral artery, and homologous to the alar foramen (foramen alare) observed in some modern artiodactyls such as Bos and perissodactyls as Equus (Aurich et al. 2020). The alar foramen is possibly derived from an alar notch that is closed anteriorly before reaching the occipital facets. In that sense, the illustration in (Evans and Lahunta 2012: fig. 4–50) of the atlas of Canis showing an alar foramen would be incorrect, and the foramen indicated as such is in reality the transverse foramen (foramen transversarium; Aurich et al. 2020).
A transverse foramen is present in the atlas of M. saladensis. The posterior opening of transverse foramen is evident in posterior view (Fig. 5b), lateral to the facets for the axis. The anterior opening of the transverse foramen is positioned ventrally approximately at the anteroposterior midpoint of the atlas body where a small exit is visible (Fig. 5b, d,e). This anterior exit of the transverse canal is posterior to and distinct from the alar notch, and would likely give passage to vessels including the vertebral artery and veins as has been observed in several modern placental mammals including carnivorans like Canis, primates like Homo, and perissodactyls like Equus (Sisson, 1914; Evans and Lahunta 2012; Standring 2015; Constantinescu et al. 2018). The posterior position of the posterior opening of the transverse foramen is similar to other ungulates like the perissodactylamorph Cambaytherium (Rose et al. 2019), the perissodactyl Hyracotherium (Wood et al. 2011), hegetotheriid notoungulates like Hegetotherium and Pachyrukhos (Sinclair 1909), the interatheriid notoungulate Interatherium (Sinclair 1909), the leontiniid notoungulate Gualta cuyana (Cerdeño and Vera 2015), the xenungulate Carodnia vierai (Paula Couto 1978), the tylopod artiodactyl Lama glama (IMNH R-2392), among others. However, in M. saladensis the transverse canal is elongated in comparison to the abovementioned taxa, which generates an anteriorly located anterior opening of the transverse foramen. It is interesting to note that in modern ruminant artiodactyls, the transverse foramen is absent (Aurich et al. 2020).
Posterodorsal to the occipital facet there is a lateral foramen (foramen vertebrale laterale), for the first cervical spinal nerve and vertebral artery as in Canis and other mammals (Evans and Lahunta 2012; Aurich et al. 2020). In SGO.PV.21700, it is only possible to see the exit of the lateral foramina in the ventral aspect of the dorsal arch (Fig. 5f), as the dorsal entry is still covered in sediment (Fig. 5c). The vertebral foramen of the atlas is slightly mediolaterally wider than dorsoventrally higher (Table S2 in Online Resource 1). However, the strong interior borders of the occipital facet invading the vertebral foramen, give the foramen a pyriform shape in anterior view (Fig. 5a, b).
When we compare the anatomy of the atlas of M. saladensis (SGO.PV.21700) with the atlas of other macraucheniids like Cramauchenia (MLP 83-III-2–1), Theosodon (YPM VPPU 15164; MACN A 9255), an early Pliocene macraucheniine from Quequén Salado River (MACN PV 9779) and Macrauchenia (PIMUZ A/V 5700), most of the description given for the former fits with the anatomical features of the four latter (Fig. 5g-i). However, in Cramauchenia (MLP 83-III-2–1) and Theosodon (YPM VPPU 15164; MACN A 9255), the posteriormost portion of the articular facets for the axis are located posterior of the transverse processes and separated from them by a notch (Fig. 5g, h), which is different to the condition seen in M. saladensis, the Quequén Salado River macraucheniine (MACN PV 9779) and Macrauchenia (PIMUZ A/V 5700). In the latter three taxa, the transverse processes extend posterior to the articular facets for the axis (Fig. 5c-e, i).
At this point it is important to note a previous description and illustrations of the atlas of Macrauchenia (MACN PV 2) which describes it as similar to the atlas of ruminants because its general shape and the presence of two closely located vertebroarterial foramina (Burmeister 1864b: pPl. IV). However, this is not the case for Macrauchenia (PIMUZ A/V 5700), and close examination of this specimen suggests a misidentification in Burmeister (1864b) for several reasons. First, MACN PV 2 atlas and ruminant artiodactyls do not have a transverse foramen (Aurich et al. 2020; Fig. S1a, d in Online Resource 5), while a transverse foramen is clearly present in Macrauchenia (PIMUZ A/V 5700) and other macraucheniids (Fig. 5). Second, MACN PV 2 atlas shows a marked atlantic fossa (fossa atlantis) typical for modern ruminants (Aurich et al. 2020; Fig. S1b in Online Resource 5), but absent in Macrauchenia (PIMUZ A/V 5700) and other macraucheniids such as M. saladensis, Cramauchenia (MLP 83-III-2–1) and Theosodon (MACN A 9255; Fig. 5e, g-i). Third, the transverse process in the MACN PV 2 atlas is anteroposteriorly extended anteriorly to the occipital facet of the atlas (Fig. S1a, b in Online Resource 5), whereas in Macrauchenia (PIMUZ A/V 5700) and other macraucheniids (Fig. 5), it terminates at the base of the process for the occipital facet, creating an alar notch which is absent in MACN PV 2 atlas. The foramina in the MACN PV 2 atlas include an alar foramen ventrally (Fig. S1b in Online Resource 5), and dorsally, the dorsal exit of the alar foramen and adjacent to it, the lateral vertebral foramen (Fig. S1a in Online Resource 5), which again is the observed condition in extant ruminants but not comparable to Macrauchenia (PIMUZ A/V 5700) and other macraucheniids. In addition, the MACN PV 2 atlas shows a very strong dorsal tubercle, closely appressed articular facets for the axis and a dorsoventrally compressed ovoid vertebral foramen (Fig. S1c in Online Resource 5), among other features clearly not present in Macrauchenia (PIMUZ A/V 5700). In sum, we assert that the MACN PV 2 atlas (also illustrated in Burmeister [1864b]) is not from Macrauchenia, being instead of a ruminant, and likely, a bovid (possibly Bos or Bison) considering its specific anatomical features, size, and general shape (see Fig. S1 in Online Resource 5). Furthermore, we think that the similarities found in Lessa (1992) between MACN PV 2 atlas and the atlas of Xenorhinotherium (MCL 2644/53), and the mention of both being similar to ruminants, is likely to be an error derived from following the misidentification in Burmeister (1864b). Indeed, when we look at a dorsoventral photograph of Xenorhinotherium (Lessa 1992: pl. XIX), the atlas looks almost identical to Macrauchenia (PIMUZ A/V 5700), showing clear anterior exits of the transverse foramina in the transverse processes and alar notches posterior of the occipital facets. It is likely that Scott (1910) based his comparison for the atlas of Theosodon on the illustration of Burmeister (1864b) for Macrauchenia, because he mentions at least a couple of features like the absence of transverse foramina and anteriorly elongated traverse processes with alar foramina instead of alar notches, as key differences between both taxa. As we saw in Macrauchenia (PIMUZ A/V 5700), these alleged differences are incorrect.
In terms of relative size, when we compare the relative dorsoventral height versus the total length (ML/MH), the atlas of M. saladensis (SGO.PV.21700) is more similar to the atlas of Macrauchenia (PIMUZ A/V 5700) and the Quequén Salado River macraucheniine (MACN PV 9779), with values of 1.05, 0.99, and 1.18 respectively, being dorsoventrally higher than the atlas of Theosodon garretorum (YPM VPPU 15164) with a value of 1.43. Overall, considering shape, measurements proportions and anatomy, the atlas of M. saladensis is like a miniature version of the atlas of Macrauchenia. It is important to note here that the atlas of Cramauchenia (MLP 83-III-2–1; Fig. 5d) pertains to a subadult specimen as M2 was still erupting (not figured). The Quequén Salado River macraucheniine atlas (MACN PV 9779) could also be a subadult as its proportions seem to be a bit different to other macraucheniines as M. saladensis (SGO.PV.21700) and Macrauchenia (PIMUZ A/V 5700), in particular the relative length of its traverse processes (ML/TL) with a value of 1.58 versus 1.19 and 1.29, respectively for the latter two (Table S2 in Online Resource 1). In addition, it is interesting to note that considering the fossil records of the Quequén Salado River basin (Beilinson et al. 2017), the macraucheniid from Quequén Salado River found between Indio Rico and Cascada Cifuentes (early Pliocene; Prevosti et al. 2021) would be the first occurrence of this subfamily and of any litoptern recorded in that unit.
Axis The axis of Micrauchenia saladensis only preserves its anterior portion. The anterior articular facets for atlas are slightly mediolaterally broader than dorsoventrally deep, and strongly convex curving dorsoposteriorly (Fig. 6). On the midline of the axis, separated from the atlantic facets by sulci, there is a slightly elongated odontoid process or dens with a mediolaterally extended articular facet for the atlas. As in Theosodon and Macrauchenia, the odontoid process has a peg-like shape, which is uncommon for long-necked ungulates such as equids and camelids (Scott 1910). A keel is present on the dorsal surface of the body of the axis of M. saladensis, that runs posteriorly from the neck of the odontoid process towards the vertebral foramen (not preserved), a feature seen also in Theosodon (YPM VPPU 30601) and Macrauchenia (MUHNCAL-KM 004; PIMUZ A/V 5700; Fig. 6b).
The relative dimensions of the anterior articular atlantic facets of M. saladensis are more dorsoventrally compressed than Theosodon (YPM VPPU 30601) but less compressed than Macrauchenia (MUHNCAL-KM 004; PIMUZ A/V 5700) with a width to height ratio (WA/HA) of 0.96 for the ratio for M. saladensis versus 0.68 and 1.13 in Theosodon and Macrauchenia respectively (Table S2 in Online Resource 1). This is evident in an anterior view of the anterior articular facets for the atlas: in Theosodon, the facets are ovoid or almost circularly shaped; in Macrauchenia the facets are rectangular in shape, and in M. saladensis the facets are also rectangular shaped although less dorsoventrally compressed than in Macrauchenia (Fig. 6a, d). In addition, the odontoid process of the axis of M. saladensis is relatively shorter than Theosodon (YPM VPPU 30601) with a ratio of 1.45 between the width of the atlantic facets and odontoid process length (WA/OL) versus 0.80 in Theosodon (Table S2 in Online Resource 1). In contrast, the odontoid process of Macrauchenia has a similar relative length (WA/OL) to M. saladensis (MUHNCAL-KM 004 = 1.43; PIMUZ A/V 5700 = 1.57).
It is interesting to note that the specimen referred to Theosodon sp. from the middle Miocene Fitzcarrald area western Amazonia in Peru (Tejada-Lara et al. 2015), has noticeable differences to Santacrucian specimens of Theosodon in terms of the proportions of the anterior articular facets for the atlas and the relative length of the odontoid process, with more dorsoventrally compressed anterior articular facets for the atlas and a considerably shorter odontoid process (WA/HA = 0.84, WA/OL = 1.38; Table S2 in Online Resource 1) when compared to YPM VPPU 30601, an early Miocene (Santacrucian SALMA) specimen of Theosodon. In fact, the relative length of the odontoid process (WA/OL) puts the Fitzcarrald specimen closer to M. saladensis and Macrauchenia than to Theosodon. This would suggest that a taxonomic reinterpretation of the macraucheniid material from the Fitzcarrald area may be needed, although a taxonomic refinement could be difficult due to the limited and fragmentary condition of the material.
Fifth cervical vertebra (C5) C5 only preserves its posterior portion, so the prezygapophyses and the anterior transverse processes are missing (Fig. 7a-f). The C5 centrum is dorsoventrally compressed, and on its ventral surface, two oblique keels are present. Based on what is preserved it is likely the two keels converged anteriorly (Fig. 7e). It is interesting to mention that Scott (1910) noted that the fourth cervical vertebra (C4) of Macrauchenia presents a single ventral keel that does not diverge posteriorly into two sections in contrast to Theosodon that presents clear posteriorly divergent oblique keels that are connected anteriorly. However, close observation of Macrauchenia (PIMUZ A/V 5700, personal observation [H.P.P.]) shows that all these three cervical vertebrae (C3–C5) present two posterior oblique keels, from which only C3 completely connects with the anterior central keel as in Theosodon (Scott 1910). The postzygapophyses of C5 are prominent and projected posteriorly, forming a V-shaped profile in dorsal view (Fig. 7c).
Dorsally, it presents a mediolaterally thin and dorsoventrally low spinous process that increases in height anteriorly (Fig. 7a-d, f). The transverse processes are located approximately at the dorsoventral midpoint of the centrum, being slightly ventrally inclined. The lateral border of the transverse process is ventrally expanded (Fig. 7a, b). The transverse foramen (foramen transversarium) is not in the transverse process as in most mammals, being instead in the medial side of the wall of the neural arch (Fig. 7b, f). A similar position of the transverse foramina has been observed in other macraucheniids, Theosodon (YPM VPPU 15164) and Macrauchenia (NHMUK PV M 43,402 A-B), and is also seen modern camelids (Owen 1838). From the two transverse foramina, only the right one was completely preserved in SGO.PV.21700. The vertebral foramen of C5 is circular in distal view (Fig. 7b).
All these features are in general terms consistent with the C3–C5 in Theosodon (Scott 1910) and Macrauchenia (PIMUZ A/V 5700), which are the elongated vertebrae of the cervical series apart from the axis. However, there are some particular features that allow us to confidently assign it to C5. First, the ratio between the maximum mediolateral width at posterior end of vertebral body (WPB) and the mediolateral width between postzygapophyses (WPZ; WPB/WPZ) for the C5 of Micrauchenia saladensis is 1.30, which is similar to Macrauchenia (PIMUZ A/V 5700) which has a ratio 1.39, in contrast to the values of 1.60 and 1.54 for C3 and C4 of Macrauchenia (Table S2 in Online Resource 1). Second, the V-shaped profile of the postzygapophyses in dorsal view is only present in C5 of Macrauchenia (PIMUZ A/V 5700). On C3 and C4 of Macrauchenia (PIMUZ A/V 5700), the postzygapophyses are not as broadly separated and therefore the incisure between the facets is not as deep. In Theosodon this feature seems to be present (Scott 1910: pl. XVIII fig. 4a) although it is less developed than in Macrauchenia and M. saladensis, as the angle between the postzygapophyses is posterior to the beginning of the body. In addition, in the M. saladensis specimen the posterior transverse processes do not reach the posterior margin of the body, a feature present in C5 of Theosodon (see Scott 1910: pl XVIII, fig. 4-4a) and Macrauchenia (PIMUZ A/V 5700). In C3 and C4 of the latter taxon, the posterior transverse processes reach the margin of the body posteriorly, and their lateralmost tips even surpass the margin of the body posteriorly.
Seventh cervical vertebra (C7) The C7 of Micrauchenia saladensis is short and robust (Fig. 7g-l). C7 is well preserved, although the anterior portion of the vertebral arch with the prezygapophyses is missing. In anterior view, the body is ovoid in shape being slightly dorsoventrally compressed (Fig. 7g), while in posterior view, the body is semilunar in shape with a flattened ventral border (Fig. 7h). The posterior end of the body is more strongly dorsoventrally compressed and relatively wider than the anterior portion. This is partially due to the presence of large and concave posterior costal foveae or demifacets (Fovea costalis caudalis; Fig. 7h) for the articular surface of the head of the first rib (caput costae). Ventrally, there is a strong keel that widens mediolaterally and dorsoventrally thickens as it approaches the anterior margin of the vertebral body, causing the anterior body face to be ventrally expanded at the level of the keel (Fig. 7g, h, k).
The postzygapophyses are widely separated and short (Fig. 7g-i), compared to the posterior position and closeness of the postzygapophyses on C5 (Fig. 7b, c). Anterior to the postzygapophyses, there is a low spinous process that appears to increase in height anteriorly (Fig. 7j, l). The transverse processes are dorsoventrally thick and anteroposteriorly short with the anterior margin projecting slightly anterior to the anterior end of the vertebral body (Fig. 7i-l). Dorsal of the main tubercle of the transverse process, there is a tiny tubercle pointing anteriorly (Fig. 7g, j). Similar to most mammals (Rose 2006), including Macrauchenia (PIMUZ A/V 5700), there is no transverse foramen on C7. In Theosodon, tiny transverse foramina were described at the base of the transverse processes, although too small to accommodate the major trunk of the vertebral artery (Scott 1910).
Overall, these features are comparable with the anatomy of C7 in Macrauchenia (PIMUZ A/V 5700) and what has been previously described for the C7 of Theosodon (Scott 1910). However, there are some subtle anatomical differences in the C7 of M. saladensis that differentiate it from Macrauchenia (PIMUZ A/V 5700). First, in the anteriormost portion of the vertebral body, the ventral keel of Macrauchenia is more mediolaterally compressed than in M. saladensis. Second, the transverse processes in M. saladensis have a small posterior projection (Fig. 7i-k), whereas in Macrauchenia a posterior projection is absent/reduced.
In terms of C7 general proportions, M. saladensis is very similar to Macrauchenia (PIMUZ A/V 5700) with a WPB/WPZ ratio of 1.08 and 1.07 respectively, which is substantially different from any other measured vertebrae of the cervical series (Table S2 in Online Resource 1). The rest of the calculated ratios are fairly similar between these two species, with the exception of the maximum anteroposterior vertebral body length versus maximum mediolateral width at anterior end of vertebral body ratio (LB/WAB), with values of 1.17 in M. saladensis and 1.05 in Macrauchenia, which means that C7 is relatively longer in the former than in the latter. Theosodon lallemanti (YPM VPPU 15216) and one specimen of Xenorhinotherium bahiense (MCL 2643/88) have a LB/WAB ratio of 1.16 and 1.18 respectively, and are very similar to M. saladensis. However, there seems to be some variation in the relative length even within the same genus or species, as Theosodon garretorum (YPM VPPU 15164) has a LB/WAB ratio of 1.07 which puts it closer to Macrauchenia (PIMUZ A/V 5700), and Xenorhinotherium shows a wide range of intraspecific variation with LB/WAB values between 1.18 and 1.49 (Table S2 in Online Resource 1).
Second thoracic vertebra (T2) SGO.PV.21700 includes a vertebra that we tentatively identify as the T2 of Micrauchenia saladensis (Fig. 8). The postzygapophyses are not preserved, as the posterior part of the vertebral arch is missing. Only the left prezygapophysis is preserved. This vertebra is very similar to C7 in its general shape, although is considerably anteroposteriorly shorter (Fig. 8c-f). Apart from the length, the most notable difference is the presence of anterior costal foveae (fovea costalis cranialis) for the head of the second rib and costal foveae for the articular surface of the second rib tubercle (tuberculum costae) on the transverse process (fovea costalis processus transversi; Fig. 8a).
Overall, the vertebral body of T2 in M. saladensis is dorsoventrally compressed, however, the posterior aspect is slightly more compressed than the anterior aspect (Fig. 8a, b). The anterior costal foveae are mostly concave and located lateroventrally on the vertebral body (Fig. 8a). Adjacent to the medial margin of each fovea, there is a relatively small but deep foramen which was likely a nutrient foramen for the entrance of blood vessels to innervate the vertebra (Evans and Lahunta 2012). Even though Scott (1910) did not mention this nutrient foramen for Theosodon on any of the thoracic vertebra, his drawing of the anterior view of T1 shows what it seems to be a couple of nutrient foramina in a similar position to M. saladensis. In Macrauchenia (PIMUZ A/V 5700) there seem to be similar canals in the same position on T2 although relatively smaller in size.
The posterior costal foveae of T2 in M. saladensis are also concave and located lateroventrally of the vertebral body, but they do not present nutrient foramina as the anterior coastal foveae (Fig. 8b). Ventrally, the anterior and posterior costal foveae are close to each other due to the anteroposterior compression of the vertebral body (Fig. 8d). In the same ventral aspect, along the median plane of the vertebral body of T2, there is an anteroposterior keel. This keel is considerably weaker than the keel on C7. In contrast, T2 in Macrauchenia (PIMUZ A/V 5700) does not present a clear ventral keel.
The prezygapophysis of T2 in M. saladensis is concave and positioned dorsal to the transverse process with its anteriormost portion slightly surpassing the anterior end of the vertebral body. Dorsally, there is a relatively developed spinous process which is poorly preserved, so it is not possible to estimate its total length and height (Fig. 8a-c, e, f). The spinous process is bent posteriorly and its basal width suggests that there is a substantial portion missing, and it is likely that this missing portion extended well posterior of the posteriormost margin of the vertebral body. The posterior inclination and presumable large size of the spinous process suggests that this vertebra is T2 instead of the first thoracic vertebra (T1), considering that in Theosodon (YPM VPPU 15164) and Macrauchenia (PIMUZ A/V 5700) T1 has a base that is not inclined posteriorly. T3 and the following thoracic vertebrae can be discarded as in Theosodon (YPM VPPU 15164) and Macrauchenia (PIMUZ A/V 5700) they show clear differences with T1 and T2, having shorter traverse processes, very robust and high neural spines and laterally oriented costal foveae in the transverse processes.
The transverse processes are dorsoventrally higher and anteroposteriorly shorter than C7 (Table S2 in Online Resource 1), presenting ventrally concave costal foveae for the for the second rib tubercles which have a slight anterolateral orientation (Fig. 8d-f). The transverse processes are continuously extended from their costal foveae ventrally to the prezygapophyses dorsally, which it also suggests a T2 identity, as this is similar to what is observed in this vertebra from Macrauchenia (PIMUZ A/V 5700). In contrast, T1 in Macrauchenia (PIMUZ A/V 5700) presents a mediolateral constriction that separates the transverse processes from the prezygapophysis. This constriction is also observed in the T1 of Theosodon (Scott 1910: pl. XVIII, fig. 6), although in this case, the separation is even clearer as there is more distance between the transverse processes and the prezygapophyses. The oblique ventrolateral orientation of the costal foveae of the transverse processes in M. saladensis is similar to the same in T2 of Macrauchenia (PIMUZ A/V 5700) which also supports a T2 identity, whereas in the latter genus, the costal foveae on T1 are oriented fully ventrally. Interestingly, Scott (1910) describes T1 in Theosodon as having a ventrally oriented costal foveae of the transverse processes, although the T1 drawing in his monograph (pl. XVIII, fig. 6) shows an anteriorly oriented costal foveae of the transverse processes.
In terms of relative proportions, T2 of M. saladensis is largely similar to Macrauchenia (PIMUZ A/V 5700) although some differences are evident due to the wider posterior end of vertebral body (WPB) in the latter (Table S2 in Online Resource 1).
Thoracic limbs
Scapula The left scapula of SGO.PV.21700 is fragmentary, only preserving part of its posterodorsal portion which is heavily damaged and eroded (Fig. 9a). Only the lateral surface is exposed, and due to its fragility it is not possible to fully remove the sediment from the medial surface without compromising the entire element. The specimen preserves a section of the scapular spine (spina scapulae), in which the dorsal tubercle, as seen in other macraucheniids (e.g., Theosodon [YPM VPPU 15164]), has been probably eroded away. Anteriorly to the scapular spine, there is a small portion of the fossa supraspinata. Posteriorly to the scapular spine is a portion of the fossa infraspinata with part of the dorsal and posterodistal border of the scapula preserved. The preserved parts of this element are very similar to other macraucheniids, including Theosodon (Scott 1910: pl XIX, fig. 1), Macrauchenia (PIMUZ A/V 5700), and Xenorhinotherium (Lessa 1992: pl. XXIV), although the state of preservation does not allow more detailed comparison.
Ulna-radius The ulna and radius of Micrauchenia are coosified, so they are here described as one element (Figs. 9b-e and 10a). The proximal end and part of the proximal portion of the diaphysis were not preserved, but the ulna and radius were likely fully coosified along their entire length considering this condition is observed in other macraucheniines (e.g., Promacrauchenia [MACN PV 5710] and Macrauchenia [MACN PV 2]).
The coosification or fusion of the ulna-radius strongly suggests that M. saladensis is a member of the subfamily Macraucheniinae sensu Soria (1981, 2001). Macraucheniids included in this subfamily in which these elements are known such as Promacrauchenia, Macrauchenia and Xenorhinotherium possess a fully fused ulna and radius in adult specimens (Gervais 1855; Parodi 1931; Cartelle and Lessa 1988), in contrast with earlier macraucheniids (“Cramaucheniinae”) such as Theosodon and Llullataruca, that display the ulna and radius in close contact but without any coosification (Scott 1910; McGrath et al. 2018).
A well-developed medial aliform expansion is present on the radius diaphysis of M. saladensis (Fig. 9b-d), and proximal from it, there is marked bend towards the lateral aspect of the element. Both features further suggest an affinity between M. saladensis and macraucheniines such as Macrauchenia (MACN PV 2) and Promacrauchenia (MACN PV 9526). The aliform expansion is formed by an arched medial thickening of the radius which is particularly rugose along its posterior aspect. This expansion has been observed to different degrees of development in Promacrauchenia, Macrauchenia and Xenorhinotherium (Parodi, 1931; Lessa, 1992). Considering its position, the rugose area on the aliform expansion could have provided attachment to the radial head (caput radiale) of the deep digital flexor muscle (M. flexor digitorum profundus; Sisson 1914; Denoix 2019; Aurich et al. 2020; MacLaren and McHorse 2020; Blanco et al. 2021). This is different from the condition present in earlier macraucheniids as Theosodon (YPM VPPU 15164; MACN A 2569–2608; MACN A 9253), in which the radius does not exhibit this extreme protrusion in its diaphysis. However, Theosodon gracilis (MACN A 2569–2608) has a relatively subtle thickening in a similar area of the diaphysis, which could be considered a precursor of the aliform expansion seen in more derived macraucheniids.
M. saladensis shows a particularly broad aliform expansion, so much so that the maximum mediolateral width of the ulna-radius is greatest at the level of the aliform expansion, rather than the distal epiphysis (53.28 vs 43.95 mm, respectively; Table S3 in Online Resource 1). When we calculate the ratio between the maximum mediolateral width of the aliform expansion and the maximum mediolateral width of the distal epiphysis for different macraucheniids (URA/URD; Table S3 in Online Resource 1), M. saladensis (SGO.PV.21700) has a value of 1.21, similar to Macrauchenia patachonica (MACN 2; PIMUZ A/V 5700; MNHN.F.PAM75) and MNHN-Bol-V 003372 described by Anaya and MacFadden (1995) as Promacrauchenia sp. with values of 1.08–1.31 and 1.12, respectively. In contrast, Promacrauchenia antiquua (MACN PV 5710) and Promacrauchenia kraglievichi (MACN PV 9526) have values below 1 of 0.83 and 0.93, respectively.
In M. saladensis the aliform expansion is positioned towards the distal end of the radius (Fig. 9b-d). The position of the expansion in M. saladensis is not as distal as the condition in Macrauchenia patachonica but is considerably more distal than that observed in Promacrauchenia sp (MNHN-Bol-V 003372) where the expansion is located on the proximal half of the radius (Anaya and MacFadden 1995). Due to the missing proximal half in the ulna-radius of SGO.PV.21700, it is not possible to quantify how far distal the aliform expansion is positioned in M. saladensis relative to the total radius length.
The distal radial epiphysis exhibits two articular facets for the scaphoid and the lunate (Fig. 10a). The scaphoid facet is positioned medially and is approximately twice the mediolateral width of the lunate facet. The maximum mediolateral width and anteroposterior length of the scaphoid facet are approximately subequal and the articular surface is anteriorly concave and posteriorly convex. The posterior margin of the scaphoid facets extends further posterior relative to the posterior margin of the lunate facet. The lunate facet is anteroposteriorly longer than mediolaterally wide. Similar to the scaphoid facet, the lunate facet is anteriorly concave and posteriorly convex. Lateral to the lunate facet, on the distal surface of the ulna, there is a concave cuneiform facet (Figs. 9e and 10a). The posterolateral part of the facet is damaged. The preserved has a similar mediolateral width than the lunate facet. The pisiform facet on the ulna, observed in other macraucheniids, was not preserved in SGO.PV.21700 due to damage.
In terms of absolute size, the ulna-radius of M. saladensis (SGO.PV.21700) is smaller Theosodon garrettorum (YPM VPPU 15164), Promacrauchenia antiquua (MACN PV 5710), Promacrauchenia kraglievichi (MACN PV 9526) and Macrauchenia patachonica (MACN 2; PIMUZ A/V 5700; MNHN.F.PAM75; Table S3 in Online Resource 1), although due to the lack of the proximal end it was not possible to compare the total length of the element. Two specimens, MLP 57-X-10–157 and MLP 91-III-1–26 (Macraucheniinae indet.), from the late Miocene and early Pliocene respectively, are of similar size to SGO.PV.21700 (Table S3 in Online Resource 1). However, they do not have a clear aliform expansion of the radius as in SGO.PV.21700.
Carpals
The preserved elements of the carpus of Micrauchenia saladensis (SGO.PV.21700) include the scaphoid, lunate, cuneiform, magnum, and unciform and pisiform (Figs. 9b, c, 10b, c, 11a-f, 12a-f, 13a-f, 14a-f, 15a-f and 16a-f). The trapezium and trapezoid were present in life but are not preserved with the specimen described. The centrale is absent as a discrete bony element (i.e., probably fused with the scaphoid). Overall, the articulated carpus of M. saladensis is almost twice as mediolaterally wide than anteroposteriorly long (Fig. 10b, c; APL/MLW = 0.47; Table S4 in Online Resource 1).
In terms of absolute size, the carpals of M. saladensis are of similar size than Cramauchenia normalis, considerably smaller than Theosodon (~31%; 8–60%), Cullinia cf. C. levis (~40%), Promacrauchenia antiquua (~65%), Macrauchenia patachonica (132%), and Xenorhinotherium bahiense (123%; Table S4 in Online Resource 1). Interestingly, the magnum of Coniopternium primitivum is slightly smaller but its lunate is slightly larger than in M. saladensis. However, considering our small sample for comparisons, it is entirely possible that in a bigger sample, the carpal size distributions of Coniopternium primitivum and M. saladensis could overlap.
In terms of general proportions, M. saladensis’s articulated carpus falls within the range observed in other macraucheniids (APL/MLW = 0.46—0.51; Table S4 in Online Resource 1), being most similar to Macrauchenia patachonica (MNHN.F.PAM75; APL/MLW = 0.46; Table S4 in Online Resource 1).
Scaphoid Among the proximal row of carpals, the scaphoid (os scaphoideum) is proximodistally short, mediolaterally wide and anteroposteriorly deep, presenting a distinctive hook-shaped palmar tuberosity posteriorly (Figs. 9b, c, 10b, c and 11a-f and Table S4 in Online Resource 1). There is a single proximal facet for the radius, which is largely convex on its anterior half, and concave in the posterior half towards the palmar tuberosity (Figs. 10b and 11a-c, e, f). The medialmost portion of this facet is missing due to the preservation of the element. Distally the scaphoid has three facets for the trapezium, trapezoid and magnum, ordered from medial to lateral (Figs. 10c and 11d-f). Only a posterior portion of the facet for the trapezium is preserved. The articular surface is concave and oriented distally. The trapezoid facet is the largest of the three facets. The articular surface faces distally and is overall slightly concave. The magnum facet is weakly convex anteriorly and concave posteriorly. The articular surface of the magnum is oriented distolaterally (Fig. 11f). Given the shape and position of this distolateral portion that extends over the magnum, we hypothesize that the centrale has fused with the scaphoid (Holmgren 1952). On the lateral surface of the scaphoid there are two facets for the lunate, one proximal next to radius facet and, one distal next to the magnum facet. The distal facet is somewhat bigger than the proximal, and both facets are proximodistally thin but anteroposteriorly elongated.
The general configuration of the scaphoid in M. saladensis is overall similar to Cramauchenia (MNHN.F.COL188; MNHN.F.COL189), Theosodon (MLP 12–850; MLP 12–883; Fig. 11g, h), Macrauchenia (MLP 12–1660; MNHN.F.PAM75; Fig. 11k, l), Xenorhinotherium (MCL specimens), and also to the late Miocene macraucheniid MLP 57-X-10–157 (Macraucheniinae indet.; Fig. 11i, j). However, there are some differences between macraucheniines including M. saladensis with earlier macraucheniids (e.g., Cramauchenia). First, the trapezoid facet in Cramauchenia (MNHN.F.COL188; MNHN.F.COL189) and Theosodon (MLP 12–850; MLP 12–883) is divided into two concavities separated by a ridge of which the anterior concavity is mediolaterally wider (Fig. 11g). In contrast, in M. saladensis, Macrauchenia (MLP 12–1660; MNHN.F.PAM75) and MLP 57-X-10–157 (Macraucheniinae indet.), the trapezoid facet of the scaphoid is a continuous surface without any clear ridge separating the anterior and posterior portions of the facet (Fig. 11d, i, k).
Another difference is that in M. saladensis, Macrauchenia (MLP 12–1660; MNHN.F.PAM75) and MLP 57-X-10–157 (Macraucheniinae indet.), the magnum facet in distal view has a similar mediolateral width throughout its length giving it a largely rectangular shape. In Cramauchenia (MNHN.F.COL188; MNHN.F.COL189) and Theosodon (MLP 12–883) the magnum facet is mediolaterally wider in its anterior aspect than its posterior aspect which confers it a pyriform shape. However, in Cramauchenia (MNHN.F.COL188; MNHN.F.COL189) the magnum facet is much shorter than in Theosodon (MLP 12–883), being most of the facet in the anterior half of the scaphoid. Additionally, in M. saladensis (SGO.PV.21700) and MLP 57-X-10–157 (Macraucheniinae indet.) the distal facet for the lunate in the scaphoid is anteroposteriorly elongated, having approximately the same length of the magnum facet (Fig. 11f, j), whereas in Macrauchenia (MNHN.F.PAM75), this facet is much shorter, being proximal to only the posterior portion of the magnum facet (Fig. 11l). In Cramauchenia (MNHN.F.COL188; MNHN.F.COL189) and Theosodon (MLP 12–883), the distal lunate facet has a fusiform-shaped expansion in the anterior margin of the scaphoid, proximal to the anterior portion of the magnum facet (Fig. 11h). When comparing the proximal lunate facet of the scaphoid, M. saladensis (SGO.PV.21700) is more similar to Macrauchenia (MNHN.F.PAM75) as both have a very proximodistally thin facet (Fig. 11f, l). In contrast, Theosodon (MLP 12–883), and MLP 57-X-10–157 (Macraucheniinae indet.) have a proximal lunate facet more marked and proximodistally wider (Fig. 11h, j).
In terms of relatively size, the proportions of the scaphoid of SGO.PV.21700 are very similar and within the range of other macraucheniids here compared (Table S4 in Online Resource 1).
Lunate Both the left and right lunate bones (os lunatum) of M. saladensis are preserved, although the left lunate is damaged and lacks the proximoanterior portion of the bone. The lunate is mediolaterally narrow, anteroposteriorly deep and proximodistally moderately tall (Figs. 9b, c, 10b, c and 12a-f and Table S4 in Online Resource 1). Proximally, the radial facet is anteroposteriorly elongate, being strongly convex in the anterior half and slightly concave in the posterior half (Figs. 10b and 12c, e, f). Medially, there are two proximodistally thin and anteroposteriorly elongate facets for the scaphoid (Fig. 12e). One of these facets is proximally located next to the radial facet, and the other is distally located next to the magnum facet. The outline of both facets is very subtle with poorly delimited margins probably due to some erosion of the element. Laterally, there is a proximodistally broad and slightly concave facet for the cuneiform located on the distal half of the lunate (Fig. 12f). When articulated with the cuneiform both elements are in close contact throughout their length, except at their proximoanterior aspect where a substantial gap opens up between them (Fig. 10b). On the distal surface of the lunate of M. saladensis, there is an anteroposteriorly elongate facet for the magnum. The facet is slightly constricted with a flat articular surface anteriorly and strongly concave posteriorly to form a saddle-shaped articular surface (Fig. 12d, e).
Overall, the general configuration of this element is very similar to the lunate of Coniopternium primitivum (MNHN.F.SAL1051), Cramauchenia normalis (MNHN.F.COL187; MNHN.F.COL191), Theosodon (MLP 12–853), Promacrauchenia antiquua (MACN PV 7986), and Macrauchenia (MLP 12–1660; MLP 12–1424; PIMUZ A/V 5700; MNHN.F.PAM75) and Xenorhinotherium (MCL specimens). However, there are some noticeable differences between the lunates of different macraucheniids (Fig. 12g-l). For instance, the proximal facet for the scaphoid is very marked and proximo-distally thick in Coniopternium primitivum (MNHN.F.SAL1051; Fig. 12h) and Cramauchenia normalis (MNHN.F.COL187; MNHN.F.COL191), in contrast to M. saladensis (SGO.PV.21700), Promacrauchenia antiquua (MACN PV 7986), and Macrauchenia (MNHN.F.PAM75), in which this facet extremely proximodistally thin (i.e., almost absent; Fig. 12e, j, l). A similar condition for this facet can be inferred for Theosodon sp. (MLP 12–883) considering the proximodistally thick proximal lunate facet present in its scaphoid (Fig. 11h).
In addition, there are some differences in the position of the distal scaphoid facet of the lunate among macraucheniids. In Coniopternium (MNHN.F.SAL1051), Cramauchenia normalis (MNHN.F.COL187; MNHN.F.COL191), and Promacrauchenia antiquua (MACN PV 7986) the distal scaphoid facet is located in the anterior margin being proximodistally thick (Fig. 12h, j), whereas in Macrauchenia (MNHN.F.PAM75) this is located posteriorly close to the posterior margin (Fig. 12l). Considering the position of the distal facet for the lunate in the scaphoid of Theosodon sp. (MLP 12–883; Fig. 11h), we can infer that its lunate is similar to the three formers in the position of this facet. The proximodistally thin and anteroposteriorly elongate facet for the scaphoid in the lunate of M. saladensis (SGO.PV.217000) is different to both previous conditions (Fig. 12e). Considering its overall shape, the lunate of M. saladensis (SGO.PV.217000) is more similar to the lunate of Macrauchenia (MNHN.F.PAM75), particularly in the marked lateral curvature of their posterior end (Fig. 12a, k).
In terms of proportions, the lunate of M. saladensis is similar to Promacrauchenia antiquua (MACN PV 7986), but somewhat mediolaterally wider than the lunate in Macrauchenia (MNHN.F.PAM75) and Xenorhinotherium (MCL specimens), with a ratio between the maximum anteroposterior length and the maximum mediolateral width (MAPL/MMLW) of ~1.9 in contrast to Xenorhinotherium and Macrauchenia which have a consistently a greater ratio (MAPL/MMLW > 2.2; Table S4 in Online Resource 1). In contrast, Coniopternium (MNHN.F.SAL1051 is somewhat mediolaterally wider than the lunate in M. saladensis, having a MAPL/MMLW of 1.75.
Cuneiform The cuneiform (os triquetrum) of M. saladensis is large and proximodistally elongate in comparison to other carpal bones (Figs. 9b, c, 10b, c and 13a-f). On the proximal surface of the cuneiform there is an anteroposteriorly narrow and strongly convex articular facet for the ulna (Figs. 10b and 13a-c, e, f). On the posterior surface there is palmar tuberosity which has an enlarged pisiform facet on the proximal surface (Fig. 13b, c, e, f). The ulnar and pisiform facets are not well delimited from one another. On the distal surface of the cuneiform there is a wide concave facet for the unciform (Figs. 10c and 13d-f). On the medial surface, there are two anteroposteriorly elongate facets for the lunate and magnum (Fig. 13e). The facet for the lunate is positioned more proximally and oriented medially. The facet for the magnum is located distally and oriented mediodistally to form a trapezoid profile. In this regard, the cuneiform of M. saladensis (SGO.PV.21700) is more similar to other macraucheniines as Promacrauchenia antiquua (MACN PV 7986), and Macrauchenia (MNHN.F.PAM75; Fig. 13j, l), in contrast to Cramauchenia normalis (MNHN.F.COL186) and Theosodon sp. (MLP 12–877; MLP 12–850) in which the articular surface for the magnum is oriented medially (Fig. 13h). In addition, Cramauchenia normalis (MNHN.F.COL186) and Theosodon sp. (MLP 12–877; MLP 12–850) have an anteroposterior elongate ridge dividing the pisiform facet of the cuneiform (Fig. 13g) in contrast to the smooth surface seen in the pisiform facet of macraucheniines (Fig. 13c, i, k).
In terms of relative size, the cuneiform of M. saladensis is within the variation seen in other macraucheniids (Table S4 in Online Resource 1).
Pisiform The pisiform of M. saladensis (os piciforme) is robust and anteroposteriorly elongate in comparison to the other carpal elements (Fig. 9c, 10b, c and 14a-f and Table S4 in Online Resource 1). Anteriorly, on the proximal half of the bone, there is a crescent-shaped facet for the ulna, and on the distal half, a larger and elongate facet for the cuneiform (Figs. 10b and 14a, c-f). The pisiform is slightly mediolaterally constricted around the mid-point of its anteroposterior axis. The anterior end is proximodistally compressed in comparison with the posterior end which is strongly proximodistally expanded (Fig. 14e, f).
Overall the pisiform of M. saladensis is very similar in shape to the pisiform of Theosodon (YPM VPPU 15164), Cullinia cf C. levis (MLP 55-IV-28–97), Promacrauchenia antiquua (MACN PV 7986), Xenorhinotherium (MCL specimens), and Macrauchenia (PIMUZ A/V 5700; MNHN.F.PAM75; Fig. 14a-f, i-n). The posterior expansion of the pisiform observed in M. saladensis is present in macraucheniines (i.e., Promacrauchenia antiquua, Macrauchenia, and Xenorhinotherium) but not Theosodon. However, even though the pisiform associate to the holotype of Cullinia levis presents a posterior expansion of the pisiform (MLP 29-IX-1–78; Fig. 14g, h), this element presents a swelling distally and anterior articular facets for the ulna and the cuneiform that differ noticeably from other macraucheniines including the pisiform of Cullinia cf C. levis (MLP 55-IV-28–97; Fig. 14i, j), which comes from the same site as the holotype (i.e., Arroyo Chasicó, Buenos Aires, Argentina). Considering these differences and also considerable difference in size between both specimens of Cullinia, it is possible that the pisiform associated to the holotype of Cullina levis (MLP 29-IX-1–78) belongs to a different taxon.
When considering relative proportions, the pisiform of M. saladensis has a relatively more proximodistally expanded posterior end than other macraucheniids (MMLW/Maximum proximodistal thickness [MPDT] = 0.77 versus MMLW/MPDT ≥ 0.82; Table S4 in Online Resource 1).
Magnum The magnum of M. saladensis (os capitatum) articulates with scaphoid, lunate and cuneiform proximally, the trapezoid medially, the unciform laterally and metacarpals II and III distally (Figs. 9b, c, 10c, and 15a-f). The scaphoid facet is robust, being anteroposteriorly elongate and mediolaterally narrow (Fig. 15a-c, e). The posterior surface of the magnum is bulbous with a proximomedially oriented convexity that articulates, in part, with the mediodistal portion of the scaphoid. If our hypothesis about the fusion of the centrale with the scaphoid in M. saladensis (and other macraucheniids) is correct, this facet would have originally articulated with the centrale. The lunate facet has a similar general shape to the scaphoid facet, but it is slightly larger and its posterior portion is even more convex where it articulates with the proximalmost part of the posterior convexity of the magnum (Fig. 15a-c, f). On the lateral surface of the magnum, there are two articular facets for the cuneiform proximally and the unciform distally (Fig. 15f). The facet for the cuneiform is mostly concave. The facet for the unciform is strongly convex anteriorly and flat posteriorly. Both facets are oriented proximolaterally.
On the distal surface of the magnum of M. saladensis, there is a wide, concave facet for Mc III (Figs. 10c and 15d). Medially adjacent to the facet for Mc III there is an anteroposteriorly elongate and concave facet for the Mc II. The facet for Mc II is somewhat medially oriented and more proximally located than the facet for Mc III. Next to the Mc II facet, separated by a ridge, there is a medially oriented facet for the trapezoid which is anteroposteriorly narrow (Fig. 15e).
The magnum of M. saladensis is most similar to Macrauchenia (MNHN.F.PAM75) in that the cuneiform and unciform facets are oriented proximolaterally and present concavities/convexities (Fig. 15a-c, k, l) whereas in Coniopternium primitivum (MNHN.F.SAL1050), Cramauchenia normalis (MNHN.F.COL192), Theosodon garretorum (YPM VPPU 15164; Scott 1910), Theosodon sp. (MLP 12–850) and Theosodon gracilis (MACN A 2569–2608) these facets face laterally with no proximal tilt so that the cuneiform and unciform do not overlay the magnum, being instead mostly flat facets (Fig. 15g-j). A similar magnum configuration as M. saladensis and Macrauchenia can be inferred for Promacrauchenia antiquua (MACN PV 7986), considering the mediodistal orientation of the facet for the magnum in its cuneiform (Fig. 13j). When comparing maximum width, length and depth ratios, the magnum of M. saladensis is very similar to other macraucheniids (Table S4 in Online Resource 1).
Unciform The unciform of M. saladensis is proximodistally elongate and mediolaterally wide with a marked palmar process on the medial half of the posterior surface (Figs. 9b, c and 16a-f and Table S4 in Online Resource 1). Proximally, there is a convex facet for the cuneiform which is subequal in anteroposterior and mediolateral dimensions (Fig. 16a-c, e, f). On the proximal half of the medial surface of the unciform there is the facet for the magnum, which is strongly concave anteriorly and flat posteriorly (Fig. 16e). A fragment of this posterior surface was not preserved. On the distal half of the medial surface of the unciform there is smaller and slightly concave facet for the Mc III. Both facets are oriented medially. Posterolaterally, adjacent to the palmar process, there is a small, shallowly concave articular facet that was likely for a vestigial Mc V (Fig. 16b, f). Among macraucheniids, a vestigial Mc V has been only described for Xenorhinotherium (Lessa, 1992: pl. XXIXa) to our knowledge, however, its presence can be inferred in M. saladensis given the presence of this well demarcated articular facet in the unciform as in Xenorhinotherium. A fifth metacarpal has been inferred in other macraucheniids including Theosodon and Macrauchenia based on the presence of a similar small facet on the posterior surface of Mc IV (Ameghino 1894b; Scott 1910; Sefve 1925). On the distal surface on the unciform of M. saladensis, there is a large and almost flat facet for the Mc IV, which it is markedly mediolaterally narrower in its posterior half relative to the anterior half (Fig. 16d).
Overall, the unciform of M. saladensis is very similar to the unciform of Cramauchenia normalis (MNHN.F.COL182; MNHN.F.COL183), Theosodon sp. (MLP specimens), Thesodon gracilis (MACN A 2569–2608), Macrauchenia (MNHN.F.PAM75), and Xenorhinotherium (MCL specimens). However, in overall shape, M. saladensis is more similar to Macrauchenia (MNHN.F.PAM75; Fig. 16a-f, k, l). As M. saladensis, Macrauchenia has a facet for the magnum strongly convex anteriorly that overlays the unciform. In Cramauchenia and Theosodon facet for the magnum in the unciform is mostly flat and has a completely medial articulation with the magnum (Fig. 16g, h). Nevertheless, the facet for the magnum in the unciform of Theosodon is more anteroposteroirly elongate than in the unciform of Cramauchenia, being in this regard more similar to the unciform of macraucheniines like M. saladensis and Macrauchenia.
In terms of proportion the unciform of M. saladensis is very similar to other macraucheniids when comparing maximum width, length and depth ratios (Table S4 in Online Resource 1).
Metacarpals
Only three metacarpals are preserved in Micrauchenia saladensis (SGO.PV.21700), a right Mc II, a fragmentary left Mc III with part of the proximal epiphysis and diaphysis missing, and a fragmentary Mc II or Mc IV preserving only part of the distal epiphysis (Figs. 9b, c and 17a-i). In general, the metacarpals of M. saladensis are proximodistally elongated and mediolaterally narrow, overall, they are very similar in shape and proportion to the metacarpals known for other macraucheniids including Theosodon and Macrauchenia (Table S5 in Online Resource 1). In terms of total length, the metacarpals of M. saladensis are the smallest in our sample, being slightly smaller than Cramauchenia normalis (~8%), and considerably smaller than Theosodon spp. (~15%), Promacrauchenia antiquua (~63%), Promacrauchenia sp. (Anaya and MacFadden 1995; ~37%), Macrauchenia patachonica (~83%), and Xenorhinotherium bahiense (~85%; Table S5 in Online Resource 1).
Metacarpal II The second metacarpal of M. saladensis II is proximodistally elongate and mediolaterally narrow, being curved medially at its distal end (Figs. 9b, c and 17a, b, e, f). The proximal end is eroded as such the articular facets for the trapezoid, trapezium, magnum, and Mc III are not well preserved. However, proximally, there is part of the facet for the trapezoid, and laterally, a very eroded proximolaterally oriented facet for the magnum and distal to it, an eroded concave crescentic facet for the Mc III and a concavity that extends distally to receive Mc III (Fig. 17b, e). On the lateral side of the proximal end of Mc II the facet for the trapezium is not well demarcated because of its poor preservation (Fig. 17a, e). These facets are very similar to Macrauchenia patachonica (MNHN.F.PAM75; Fig. 17j, k, n, o) and to earlier macraucheniids like Cramauchenia normalis (MNHN.F.DES305) and Thesodon gracilis (MACN A 2569–2608). The diaphysis of Mc II is robust when compared with Mc III. Medially, the diaphysis of Mc II it is slightly concave along its proximal half (Fig. 9b, c). The distal end of Mc II is asymmetric, whereby the medial portion of median keel is moderately mediolaterally wider than the lateral portion. The preservation of the distal end is in general good although a posterior fragment of the head is missing. On the medial and lateral sides of the distal end of Mc II there are well demarcated depressions for the attachment of the metacarpophalangeal collateral ligaments (Sisson 1914; Evans and Lahunta 2012; Denoix 2019). The lateral depression is wider and deeper than the medial one (Fig. 17a, b). These depressions are also present on the Mc II of Macrauchenia patachonica (Fig. 17j, k) showing the same morphology. The median keel is well-defined anteriorly and extends on to the posterior surface of the distal epiphysis (Figs. 9c and 17f). The posterior surface of the distal end of Mc II is not well preserved, however, it appears likely that median keel would have continued considerably to the posterior surface as observed in other macraucheniids allowing an extensive dorsopalmar movement of the toe (Owen 1838). This inference is further supported by the presence of a strong median keel on a poorly preserved fragment of a distal metacarpal. Considering the asymmetrical morphology of the bone and the development of the median keel it is likely Mc IV or Mc II (Fig. 17i). Overall, the general structure of Mc II in M. saladensis is very similar to other macraucheniids as Cramauchenia normalis (MNHN.F.DES305), Thesodon gracilis (MACN A 2569–2608) and Macrauchenia patachonica (MNHN.F.PAM75).
Comparing the relative length of Mc II using the distal mediolateral articular width (ratio DAW/TL) M. saladensis has a value of 0.18, which it is within the range observed in our sample of macraucheniids (0.13–0.23), being proportionally similar to Cramauchenia normalis (MNHN.F.DES305), Theosodon garretorum (YPM VPPU 15164) and a couple of specimens of Xenorhinotherium bahiense (MCL 3504 and MCL 3513; Table S5 in Online Resource 1).
Metacarpal III The Mc III of M. saladensis is preserved in two parts: a fragment of the lateral side of the proximal end with the medial portion completely missing, and most of the shaft with a complete distal end (Figs. 9b, c and 17c, d, g, h). Even though the two fragments do not fit due to erosion between the contacting surfaces, the size and colour allow us to confidently assign them to the same element. The Mc III of M. saladensis is more elongate, slender and straight than Mc II, being at least 17% longer (Table S5 in Online Resource 1). The lateral side of the proximal end is worn but preserves two small articular facets for Mc IV, one closer to the anterior border and slightly concave and the other closer to the posterior border which is flat (Fig. 17d). Proximally, there is a convex facet for the magnum, and lateral to this surface and closer to the dorsal aspect, there is an oblique and extremely eroded facet for the unciform (Fig. 17d, g).
The Mc III diaphysis of M. saladensis is mediolaterally narrower than the Mc II, however, its distal articular end is considerably wider than the Mc II (14% wider; Table S5 in Online Resource 1). In terms of proximodistal length of the head, Mc III only expands at the distal most end, consequently Mc II has a moderately longer head than Mc III. In addition, the head of Mc III is symmetrical (Figs. 9b, c and 17h), which is markedly different from the asymmetric head of Mc II (Figs. 9b, c and 17f). Furthermore, in contrast to Mc II, the Mc III median keel is very weak on the anterior surface of the bone but becomes stronger as it approaches the posterior side (Figs. 9b, c and 17c, d, h). On the posterior side it is strong and proximally elongate. A distinctively weak Mc III anterior medial keel has been previously observed in other macraucheniids including Macrauchenia and Theosodon (Gervais 1855; Scott 1910). As such, the distal end of Mc III is easily distinguished from Mc II and Mc IV. It is important to note here that the strong anterior aspect of the median keel illustrated by Scott (1910: pl. XIX, fig. 6) for Mc III was probably mistakenly exaggerated as observations of the actual element (YPM VPPU 015164) show a very weak anterior keel (personal observation, H.P.P.), which is in agreement with Scott’s actual description of this element and also with other specimens of Theosodon here examined (e.g., MACN A 2569–2608, MLP 12–850). Interestingly, the lack of a strong anterior component of the keel in Mc III, has also been observed in the Mt III of the late Oligocene macraucheniids (cf. ?Coniopternium spp.) from Salla, Bolivia (Shockey 1999).
On the medial and lateral sides of the head of Mc III, there are clear depressions for the attachment of the metacarpophalangeal collateral ligaments as inferred for Mc II (Sisson 1914; Evans and Lahunta 2012; Denoix 2019), although for this element the lateral and medial depressions are equally strongly pronounced. Overall, in terms of shape and general features, the Mc III of M. saladensis is very similar to other macraucheniids like Theosodon gracilis, (MACN A 2569–2608), Theosodon sp. (MLP 12–850), Promacrauchenia antiquua (MACN PV 7986) and Macrauchenia patachonica (MNHN.F.PAM75; Fig. 17l, m, p, q), and consistent with previous descriptions (e.g., Owen 1838; Scott 1910; Sefve 1925; Lessa 1992).
Comparing the relative length of Mc III, M. saladensis has a ratio DAW/TL of 0.18, which is within the range observed in our sample of macraucheniids (0.16–0.20; Table S5 in Online Resource 1).
Phalanges
Micrauchenia saladensis preserves six phalanges, of which there are two proximal, one from right digit II, and one from left digit III, and four intermediate, one from the left digit III and three from the digits II and/or IV (Figs. 9b, c, 18 and 19). The proximal phalanges are clearly distinguished from the intermediate phalanges, as they are considerably longer than the former having an elongated diaphysis (Figs. 9b, c and 18). Proximally, the proximal phalanges have a dorsopalmar concavo-convex articular surface for the metacarpals, which is interrupted at the ventral midpoint by a marked groove for receiving the medial keel of the associated metacarpal. Distally, the articular surface of the head of the proximal phalange is smooth and saddle-shaped extending dorsally and ventrally, which probably facilitated a range of dorsopalmar movement of the digits as in Macrauchenia (Owen 1838). On the lateral and medial sides of the distal end of the proximal phalanges there are well-demarcated depressions for the attachment of interphalangeal collateral ligaments (Sisson 1914; Evans and Lahunta 2012; Denoix 2019).
The intermediate phalanges of M. saladensis are short and robust, being around 40% more elongated in their mediolateral width than their proximodistal length (Figs. 9b, c and 19 and Table S6 in Online Resource 1). Apart from their length and shape, the main differences between the intermediate phalanges with the proximal phalanges are that the proximal articular surface is more markedly concavo-convex dorsopalmarly and also slightly convex mediolaterally, and there is no median groove on the proximal articular facet. Furthermore, posteriorly, close to the proximal articular surface, the intermediate phalanges have a thickened transverse flexor tuberosity with a well demarked groove that, considering its position, was likely a point of attachment for the sesamoidean ligament (ligament sesamoideum rectum) that in Equus connects the manus intermediate phalanx with the proximal sesamoid bones (Constantinescu et al. 2018; Aurich et al. 2020). As with the proximal phalanges, the distal articular surface for the proximal end of the distal phalanges is smooth and saddle-shaped, although more lengthened on the anterior side. Posteriorly, the distal end is smooth and concave where there was likely an articulation with the median or sagittal ridge of a distal sesamoid bone as in Equus (Denoix 2019; Aurich et al. 2020). In addition, the intermediate phalanges present well-demarcated depressions on the lateral and medial sides of the distal end for the attachment of interphalangeal collateral ligaments (Sisson 1914; Evans and Lahunta 2012; Denoix 2019). These are much more pronounced in digit III, and the side facing digit III of adjacent digits (i.e., the lateral side of digits II and the medial side of digit IV).
In terms of total length, the phalanges of M. saladensis are on average of similar size than Cramauchenia normalis (less than 2% difference), slightly smaller than Coniopternium andinum (~11%), and considerably smaller than Theosodon spp. (~18%), Promacrauchenia antiquua (~61%), Promacrauchenia sp. (Anaya and MacFadden 1995; ~20%), Scalabrinitherium ferreriai (20%), Macrauchenia patachonica (~87%), Xenorhinotherium bahiense (~81%), and Macraucheniopsis ensenadensis (106%; Table S6 in Online Resource 1).
Proximal phalanx of manual digit II The proximal phalanx of digit II is elongate and slender, in particular along the shaft (Figs. 9b, c and 18a-d and Table S6 in Online Resource 1). In terms of preservation, it is highly eroded on the palmar surface and the medial half of the proximal end of the bone is missing. The lateral portion of the proximal articular surface adjacent to the groove is anteroposteriorly elongate and mediolaterally narrow. The preserved part of the proximal articulation is near identical to the same portion in the same digit of Macrauchenia (PIMUZ A/V 5700; MNHN.F.PAM75; Fig. 18i). The diaphysis is constricted and approximately 30% mediolaterally narrower than the distal end (Fig. 9b, c and Table S6 in Online Resource 1). Distally, the articular surface for the intermediate phalanx is not well-preserved, the medial portion is missing and the palmar surface is very eroded (Figs. 9b, c and 18d). On the medial side of the distal end there is a depression for the attachment of one of the interphalangeal collateral ligaments (Fig. 18b). Overall, in terms of general structure, the proximal phalanx of digit II in M. saladensis is extremely similar to that of Macrauchenia (PIMUZ A/V 5700; MNHN.F.PAM75).
Comparing the relative length of the proximal phalanx of digit II using its proximal mediolateral articular width to total length (ratio PAW/TL) and its distal mediolateral articular width to total length (ratio DAW/TL), M. saladensis has values of 0.36 for both measures, which are well within the range observed in our sample of macraucheniids (PAW/TL = 0.22–0.60; DAW/TL = 0.27–0.49; Table S6 in Online Resource 1).
Proximal phalanx of manual digit III The proximal phalanx of digit III is very similar to the proximal phalange of digit II, but less elongate and more symmetrical (Figs. 9b, c and 18e-h and Table S6 in Online Resource 1). The bone is well preserved and only damaged on the medial part of the head and part of the proximal articular surface for Mc III. Proximally, the concavo-convex articular surface is symmetrical as also observed for Macrauchenia (PIMUZ A/V 5700; MNHN.F.PAM75; Figs. 18g, j). Distally, the distal articular surface for the intermediate phalanx is more anteriorly extended than in the proximal phalanx of digit II (Fig. 9b), which is similar to what is observed in Macrauchenia (PIMUZ A/V 5700; MNHN.F.PAM75). On the medial and lateral sides of the distal head, there are depressions for the attachment of interphalangeal collateral ligaments (Fig. 18e, f).
Comparing the relative length of the proximal phalanx of digit III using the PAW/TL and DAW/TL ratios, M. saladensis has values of 0.49 and 0.39 respectively, and falls within the range observed in our sample of macraucheniids (PAW/TL = 0.32–0.71; DAW/TL = 0.30–0.56; Table S6 in Online Resource 1), although M. saladensis is more slender than the biggest species Macrauchenia (PIMUZ A/V 5700; MNHN.F.PAM75; PAW/TL = 0.64–0.66; DAW/TL = 0.48–0. 51) and Xenorhinotherium (MCL specimens; PAW/TL = 0.63–0.71; DAW/TL = 0.49–0. 56).
Intermediate phalanges of manual digits II and IV M. saladensis (SGO.PV.21700) preserves three intermediate phalanges (Figs. 9b, c and 19a-g). The intermediate phalanges for digits II and IV are described together below and they are near identical mirrored copies of one another. It is important to note at this point, that due to the striking morphological similarity between the phalanges of the fore and hind limbs in macraucheniids, and also the similarity between the phalanges of digits II and IV, previous workers have found it difficult to identify specific bones if they are not found in association with their respective metapodials (Scott 1910; Lessa 1992; Schmidt 2013). Therefore, the putative left intermediate phalange of digit IV could potentially be a pedal intermediate phalanx from digit II or IV. However, considering that SGO.PV.21700 does not preserve any unequivocal posterior element we consider this possibility to be unlikely.
The intermediate phalanges of digit II and IV are asymmetrical. Their distal ends are strongly asymmetric, having on the interior side (facing at the digit III) a marked angular process with a deep and concave depression for an interphalangeal collateral ligament attachment (Fig. 19c; Sisson 1914; Evans and Lahunta 2012; Denoix 2019). In the exterior surface of the distal end (facing away from digit III), there is a subtle concavity for the attachment of an interphalangeal collateral ligament, although not as developed as in the medial side. (Fig. 19b). Overall, the general structure of the intermediate phalanges of digit II and IV is very similar to other macraucheniids as Theosodon sp. (e.g., MLP 12–757; MLP 12–758) and Macrauchenia patachonica (MNHN.F.PAM75; PIMUZ A/V 5700).
Comparing the relative length of the intermediate phalanges for digits II and IV using the PAW/TL and DAW/TL ratios, M. saladensis has values of 0.76 and 0.53 respectively and falls within the range observed in our sample of macraucheniids in terms of proximal mediolateral width but narrower in terms of distal mediolateral width (PAW/TL = 0.64–0.88; DAW/TL = 0.58–0.74; Table S6 in Online Resource 1).
Intermediate phalanx of manual digit III The intermediate phalanx of digit III is distinguished from the intermediate phalanges of digit II and IV in that it exhibits a strong symmetry along its proximodistal axis (Fig. 9b, c and 19h-l and Table S6 in Online Resource 1). The distal end exhibits two marked angular processes each with a small concavity for the attachment of interphalangeal collateral ligaments (Fig. 19i, j; Sisson 1914; Evans and Lahunta 2012; Denoix 2019). Overall, the general structure of the intermediate phalanges of digit III is very similar to other macraucheniids as Theosodon sp. (e.g., MLP 12–756) and Macrauchenia patachonica (MNHN.F.PAM75; PIMUZ A/V 5700).
Comparing the relative length of the intermediate phalanx of digit III using the PAW/TL and DAW/TL ratios, M. saladensis has values of 0.64 and 0.49, comparable to the values observed in our sample of macraucheniids (PAW/TL = 0.55–0.90; DAW/TL = 0.50–0.70; Table S6 in Online Resource 1). The biggest species, Macrauchenia patachonica (e.g., MNHN.F.TAR813) and Xenorhinotherium bahiense (MCL specimens), tend to be in proportion mediolaterally wider than M. saladensis, being close to upper limit of both ratio ranges.
Phylogenetic results
The strict consensus tree from the parsimony analysis recovers the subfamily Macraucheniinae as a monophyletic group in contrast with “cramaucheniines” which are paraphyletic (Fig. 20a). However, all the members of Macraucheniinae, including Micrauchenia saladensis, are part of an extensive polytomy that obscures assessment of the internal relationships within this group. The Bremer support values are very low and the Jackknife resampling fails to recover all the nodes (Fig. 20a). The majority rule tree shows a much better resolved subfamily Macraucheniinae than the strict consensus tree, with M. saladensis nested in a polytomy that includes Cullinia levis and Paranauchenia spp. plus a clade of Promacrauchenia calchaquiorum, Promacrauchenia antiquua, Windhausenia delacroixi, Xenorhinotherium bahiense, Promacrauchenia sp. (Anaya and MacFadden 1995), and Macraucheniopsis ensenadensis (Fig. 20b, node 10). Promacrauchenia sp. (Anaya and MacFadden 1995) is part of a polytomy that includes Macrauchenia patachonica and Macraucheniopsis ensenadensis (Fig. 20b, node 16). The two species of Promacrauchenia form a monophyletic group in node 13, similar to the two species of Paranauchenia in node 11 (Fig. 20b). Among “cramaucheniids”, Cramauchenia normalis, Coniopternium spp. and Llullataruca shockeyi are grouped in a polytomy (Fig. 20b, node 5), as was also recovered in the strict consensus tree (Fig. 20a). The effect of removing the wildcard taxon M. saladensis a posteriori from the strict consensus resolves the extensive polytomy in Macraucheniinae, returning a topology very similar to the majority rule consensus tree (Fig. S2 in Online Resource 5).
Maximum parsimony phylogenies of Macraucheniidae. a. strict consensus tree. b. 50% majority rule consensus tree. The numbers next to the nodes in a are the absolute Bremer supports. The numbers next to the nodes in b indicate the percentage in which each node was recovered from the total number of most parsimonious trees (MPTs), and the numbers in the black circles are the nodes number. Node 7 corresponds to the subfamily Macraucheniinae, here recovered as a monophyletic group. Promacrauchenia sp. are the specimens previously attributed to this genus from the Inchasi beds, Inchasi, Bolivia, mid-Pliocene, 4–3.3 Ma (MacFadden et al. 1993; Anaya and MacFadden 1995). Both phylogenies (a, b) summarize a total of 36 MPTs (89 steps; consistency index [CI] = 0.607; retention index [RI] = 0.701)
When we mapped the apomorphies in the majority rule consensus tree, the subfamily Macraucheniinae is supported by the following eight unambiguous synapomorphies: (a) nasals vestigial or absent (char. 1); (b) the premaxillae and maxillae are sutured sagittally in dorsal view (char. 8); (c) the coronal plane that passes through infraorbital foramen is anterior to M3 (char. 9); (d) the caudal border of the nasal aperture is in level with the orbit (char. 10); (e) the anterior palatal shape narrows at P2 or P3 level (char. 11); (f) labially projected parastyle on P3-P4 (char. 17); (g) deep concavities between lingual styles (parastyle-mesostyle-metastyle) on M1-M3 (char. 18); and (h) the absence of a sagittal crest (char. 31) (Table S8 in Online Resource 2). The polytomy formed by Promacrauchenia sp. (Anaya and MacFadden 1995), Macrauchenia patachonica and Macraucheniopsis ensenadensis is supported by five unambiguous synapomorphies: (a) the absence of an entoconid on m1-m2 (char. 3); (b) a strongly compressed trigonid of m1 against the talonid of p4 (char. 4); (c) a rounded outline of the premaxillary area in palatal view (char. 12); (d) the presence of a diastema between I3-C (char. 16); and (e) the absence of an entolophid on m1-m2 (char. 23).
The consensus tree of the undated Bayesian analysis agrees with the parsimony strict consensus tree in also recovering the subfamily Macraucheniinae, although it shows a better resolution for the groups present within this subfamily (Fig. 21a). However, when we compare the undated Bayesian consensus tree with the parsimony majority rule tree, the former is considerably less resolved, although in general terms they mostly agree in the position of most of the taxa with the exception of M. saladensis. This taxon is recovered in the Bayesian tree within a polytomy that includes Promacrauchenia sp. (Anaya and MacFadden 1995), Macrauchenia patachonica, and Macraucheniopsis ensenadensis. Node support is generally low, with support for the node of the subfamily Macraucheniinae being the strongest (0.94; Fig. 21a).
Bayesian phylogenies of Macraucheniidae. a. undated Bayesian analysis. b. tip-dated Bayesian analysis calibrated in Ma. In a-b, node support is indicated using Bayesian posterior probabilities, and in b, the blue node bars represent the 95% highest posterior density (HPD) for the estimated node ages. In both phylogenies (a, b), node 4 corresponds to the subfamily Macraucheniinae, recovered as a monophyletic group. Promacrauchenia sp. includes the specimens previously attributed to this genus from the Inchasi beds, Inchasi, Bolivia, mid-Pliocene, 4–3.3 Ma (MacFadden et al. 1993; Anaya and MacFadden 1995)
The consensus tree of the tip-dated Bayesian analysis shows in general similar phylogenetic affinities as the undated consensus tree (Fig. 21b), the main difference being the position of M. saladensis, which is here more basally located within the subfamily Macraucheniinae in an extensive polytomy at the base of the subfamily (Fig. 21b, node 4). Promacrauchenia sp. (Anaya and MacFadden 1995) is positioned in a polytomy with Macrauchenia patachonica and Macraucheniopsis ensenadensis (Fig. 21b, node 9), which is similar to what is seen in the parsimony majority rule consensus tree (Fig. 20b, node 16). Node support is generally higher than in the undated consensus tree, and in particular the subfamily Macraucheniinae is strongly supported (0.93; Fig. 21b, node 4). In terms of divergence-times estimations, the origin of the subfamily Macraucheniinae (Fig. 21b, node 4) was dated at 14.06 million years ago (Ma) with an uncertainty range of 19.05–10.52 Ma considering the 95% highest posterior density interval (HPD) (Table S9 in Online Resource 2). The node including Promacrauchenia sp. (Anaya and MacFadden 1995), Macrauchenia patachonica and Macraucheniopsis ensenadensis (Fig. 21b, node 9), was estimated at 4.62 Ma with an uncertainty range of 6.73–3.40 Ma considering the 95% HPD.
Discussion
Skeletal anatomy, locomotion, and paleobiological inferences
Macraucheniids are thought to have been cursorial animals sensu Stein and Casinos (1997) considering that they possess vertically oriented limbs that favoured movement in parasagittal planes. They possess a series of anatomical features considered adaptations for cursorial locomotion, these include: loss of the clavicle (allowing a greater range of parasagittal movement of the scapula), a short olecranon process on the ulna (restricts and stabilises against pronation-supination), interlocking keels and grooves in the limb joints (allowing a wide arc of parasagittal movement with reduced risk of dislocation), and block-like carpals and appressed metapodials (increases limb strength and directs loading through the limb), digit reduction (i.e., loss/reduction of digits I and V; distal limb weight reduction, increasing locomotor economy [but see McHorse et al. 2019 for alternative interpretations]), elongated metapodials and proximal phalanges (increases length of stride), among other typical cursorial features (Hildebrand and Goslow 2001; Polly 2007). The zeugopodial elements are also fused in macraucheniines. The fusion of the radius and ulna, and in particular the radial head, further restricts pronation-supination movement, stabilizing the articulation in the parasagittal plane. Fusion of the tibia and fibula and reduction of the fibula further reduces the mass of the hindlimbs (Hildebrand and Goslow 2001; Polly 2007).
Cursorial locomotion of macraucheniids has been corroborated using biomechanical indices derived from limb measurements that suggest running capabilities for Theosodon (Cassini et al., 2012) and Macrauchenia (Carrano, 1997; Fariña et al. 2005; Blanco et al. 2021), which are the most complete macraucheniids thus far found. Micrauchenia, with a vertically oriented forelimb, block-like carpals, elongate metacarpals, interlocking keels and grooves in the joints and additional stabilizing adaptations against non-parasagittal movements (more details below), is overall very similar to Macrauchenia and was most likely cursorial as well. Even macraucheniids older than Theosodon, like Cramauchenia and Coniopternium known from the Deseadan SALMA (late Oligocene) and with some associated postcranial remains (e.g., femur, tarsals and metatarsals), display postcranial features (e.g., elongated metatarsals) associated with cursorial locomotion (Shockey 1999; Dozo and Vera 2010). This suggests that the common ancestor of all macraucheniids was likely cursorial, which is not surprising if we consider that the probably pentadactyl Protolipterna, the earliest litoptern with relatively well-preserved postcranial remains, shows features consistent with cursoriality (Bastos and Bergqvist 2007; but see Cifelli 1983b for a saltatorial interpretation).
Macraucheniids have hooves which make them “ungulates” however, the possession of hooves is not necessarily the same as having a fully unguligrade foot posture. In a previous study (Carrano 1997), Macrauchenia was found to group with digitigrade mammals based on limb and tarsal measurements (i.e., femur, astragalus, calcaneum and metatarsals), in an analysis using only two categories: plantigrade (cruro-tarsal joint) and (b) digitigrade (metatarso-phalangeal joint), and excluding unguligrades. Within the digitigrade category, Carrano (1997) included subunguligrade animals that present a fleshy pad for support. When looking at the digital articular surfaces, it is possible to infer that in Macrauchenia (PIMUZ A/V 5700), Theosodon (YPM VPPU 15164) and Micrauchenia only the intermediate and ungual phalanges (unguals not preserved in Micrauchenia) were more horizontally positioned and in contact with the ground. Among these phalanges, the intermediate phalanx of digit III was probably the most horizontally positioned while in a neutral stance, considering the more dorsally extended articular surface of the proximal phalanx of digit III when compared with digits II and IV. The foot posture present in these macraucheniids would best be described as subunguligrade (sensu Carrano 1997). In the case of Macrauchenia, this hypothesis is further supported by ichnological evidence (i.e., footprints) suggesting that this taxon possessed thick palm and plantar pads in life, and a considerably more marked print for digit III (Eumacrauchenichnus patachonicus; Aramayo and de Bianco 2009; Aramayo et al. 2015).
Apart from macraucheniines, to our knowledge no other cursorial mammal, including modern and extinct ungulates, presents an aliform expansion in the distal part of the radius, which makes difficult a clear functional interpretation for this structure based on comparisons with modern taxa. Recently, Blanco et al. (2021) suggested that the mediolaterally wide radius in Macrauchenia was for the insertion of an enlarged deep digital flexor muscle (M. flexor digitorum profundus). Considering the muscular anatomy of some terrestrial extant artiodactyls and perissodactyls, such as Equus, Tapirus, Bos and Babirusa (Sisson 1914; Campbell 1936; Kneepkens et al. 1989; Denoix 2019; Aurich et al. 2020; MacLaren and McHorse 2020), we consider this a reasonable hypothesis with the caveat that none of these other taxa are particularly close relatives of litopterns.
The deep digital flexor muscle typically originates in four heads, two humeral (caput humerale), one ulnar (caput ulnare) and one radial (caput radiale) with different degrees of development depending on the taxon, and it inserts on the flexor surface of the distal phalanges (Campbell 1936; Aurich et al. 2020). We propose that the radial head of the deep digital flexor muscle was distally expanded in macraucheniines and originated from or in part from the medial aliform expansion of the radius. Functionally, the deep digital flexor muscle is a flexor of the forefoot (digital [metacarpo/interphalangeal] joint flexion; Aurich et al. 2020; MacLaren and McHorse 2020; Etienne et al. 2021), which suggests that Micrauchenia and other macraucheniines such as Promacrauchenia and Macrauchenia had a strong musculature for flexing the forelimbs.
Blanco et al. (2021) suggested that this muscular development present in the radius of Macrauchenia was an adaptation for supporting a considerable part of the body weight on the forelimbs, specifically while running with the neck in a horizontal position. Considering the flexor function of the deep digital flexor muscle and the fact that Macrauchenia did not have naturally flexed forelimbs in neutral stance, we disagree with this interpretation. Moreover, Camelus, with an elongated neck and with a relatively large body mass (i.e., 400–657 kg; Köhler-Rollefson 1991; Makhdoomi et al. 2013), does not show any particular expansion of the radius (e.g., IMNH R-1007) even though it is well known that it puts its neck in a horizontal position while running at fast speeds. An alternative hypothesis is that a strong flexor musculature was related to rapid and sudden changes of speed and/or direction, which could be useful, for instance, to avoid potential predators. Indeed, a previous study that used anteroposterior and transverse strength indicators estimated from the section modulus of the bone in parasagittal and transverse planes (i.e., IACap and IACt), showed that the femur of Macrauchenia was extremely resistant to transverse stress (Fariña et al. 2005). This was interpreted as an adaptation to cope with traverse stresses when veering in locomotion, particularly helpful to avoid predators by means of swerving and dodging (Fariña et al. 2005).
As observed for perissodactyls and most notoungulates, litopterns exhibit mesaxonic symmetry of the manus and pes through digit III (Scott 1910, 1912; Cifelli 1993). However, litopterns differ from perissodactyls and notoungulates in their specific carpal arrangement. In litopterns, the cuneiform is in contact with the magnum, which prevents contact between the lunate and the unciform. This litoptern carpal arrangement has been called “semi-taxeopod” (Scott 1910) or “reverse alternating” (Cifelli 1993), and it is present in macraucheniids and proterotheriids with further digital reduction (Scott 1910). As such, the carpal configuration and morphology of macraucheniids is highly conserved.
A reverse alternating carpus was present in earlier macraucheniids as Cramauchenia, Coniopternium (Fig. 15g, h), and it was probably present in Polymorphis, the oldest known macraucheniid, considering that proterotheriids have a similar carpal arrangement. It is unknown when this carpal arrangement was acquired as not much is known about the carpal arrangement of the earliest litopterns or even the earliest South American “condylarths” (Cifelli 1985; Bastos and Bergqvist 2007; Bergqvist 2008). Considering the carpal arrangement of early eutherians such as Juramaia sinensis (Middle to Late Jurassic; Luo et al. 2011) and Eomaia scansoria (Early Cretaceous; Ji et al. 2002) with a lunate-unciform articulation, a lunate unciform contact is likely the plesiomorphic placental condition. This inference is supported by the lunate-unciform articulation being present in different Paleogene placental ungulates such as Arctocyon (Argot 2013), Hyopsodus (Matthew 1915), and Peryptychus (Shelley et al. 2018), among others. If we consider the proposed close relationship between litopterns, notoungulates and perissodactyls (Buckley 2015; Welker et al. 2015; Westbury et al. 2017) to be true, and the fact that notoungulates and perissodactyls (including stem perissodactyls like the anthracobunian Cambaytherium [Rose et al. 2019]) exhibit a lunate-unciform articulation, then the gain of a cuneiform-magnum articulation with consequent loss of the lunate-unciform articulation could be considered a synapomorphy for Litopterna as previously suggested (Cifelli 1993). Far less clear, with the current fossil evidence, is if the mesaxonic symmetry of the manus and pes through digit III shared by these three orders is convergent or inherited from a common ancestor.
In terms of locomotion, the distinctive reverse alternating carpal arrangement of Micrauchenia and other macraucheniids entails that the scaphoid and cuneiform contact the magnum, and therefore it is possible that the medial and lateral loading during locomotion were transferred centrally towards digit III. The load increment in digit III would reduce the load on the outer digits so they could take on balance or stabilising role, a feature that could be useful in turning or swerving while running. The carpus of Micrauchenia also shows adaptations towards higher loadings during locomotion. In particular, the proximodistally expanded pisiform of Micrauchenia and other macraucheniines such as Promacrauchenia, Macrauchenia and Xenorhinotherium contrasts with the more slender pisiform in Theosodon could be related to with enlargement of muscles like the ulnar flexor muscle of the carpus (m. flexor carpi ulnaris) and the ulnar extensor muscle of the carpus (m. extensor carpi ulnaris [m. ulnaris lateralis]; Aurich et al. 2020). Both muscles flex the wrist in large artiodactyls and in equids considering their caudal path and insertion on the pisiform (in tapirids such as Tapirus, the ulnar extensor muscle of the carpus also supports extension due to its additional insertion on Mc V; Evans and Lahunta 2012; Aurich et al. 2020; MacLaren and McHorse 2020; Etienne et al. 2021). A more distally prominent pisiform as observed in Micrauchenia and other macraucheniines, increases the lever arm of the ulnar flexor muscle of the carpus which exerts flexion during locomotion (Taylor 1974; Argot 2001).
The medial and lateral depressions on the distal ends of the metacarpals and phalanges inferred for the attachment of the metacarpophalangeal and interphalangeal collateral ligaments (Sisson 1914; Evans and Lahunta 2012; Denoix 2019) are symmetrical on digit III, but strongly asymmetrical in the digits II and IV, with the depressions on digits II and IV facing digit III being more pronounced. The medial and lateral depressions for the collateral ligaments are also observed in the metatarsals of different macraucheniids (Shockey 1999). Collateral ligaments provide lateral support and stability to the joints (Standring 2015). It is probable that more pronounced collateral ligaments facing digit III could also contributed to the stability of digit III, favouring dorsopalmar/plantar movements and preventing transverse stress. In addition, Mc II and Mc IV in macraucheniids have a stronger and more anteriorly elongate median keel than Mc III. It is likely that this strong and elongate median keel on Mc II and Mc IV allowed a wide arc of parasagittal movement and with a low risk of dislocation (Hildebrand and Goslow 2001), which in turn it could give more stability to digit III (Shockey 1999).
In sum, the aforementioned features of the forearm and digits in macraucheniids are indication of adaptations for cursorial locomotion, favouring parasagittal movement and minimizing transverse stresses. The fusion of zeugopodial elements exhibited by macraucheniines can be seen as a further step towards specialization in cursorial locomotion, providing more economy of effort (Hildebrand and Goslow 2001). The same can be said about the interlocking happening between the cuneiform and unciform with the magnum in macraucheniines such as Micrauchenia and Macrauchenia, which increases limb strength and reduces risk of dislocation during locomotion. However, the aliform expansion of the radius and the well-demarcated depressions on the digits for the insertion of collateral ligaments (particularly in the marked angular processes present in the intermediate phalanges) observed in macraucheniines are unusual and lack an extant cursorial analogue, which suggests that these features could be connected to a particular behaviour that characterized this subfamily. In that context, the swerving hypothesis (Fariña et al. 2005) proposed as an adaptation of Macrauchenia against ambush predators such as Smilodon, is an interesting explanation for these macraucheniine anatomical structures. However, a small aliform expansion of the radius is present in some early macraucheniines from the late Miocene like Micrauchenia, Promacrauchenia sp. (Anaya and MacFadden 1995) and Promacrauchenia, and therefore predates the arrival of felids to South America during the GABI (Woodburne 2010; Cione et al. 2015), and long before the first appearance of large felids like Smilodon in the South American fossil record in the Ensenadan SALMA (~2.00–0.70; Manzuetti et al. 2018). The only candidates for such predation pressure could be other predators from the late Miocene in South America like Thylacosmilus atrox, a marsupial sparassodont that due to its plantigrade pes posture, strong forelimbs and forwardly placed eyes has been inferred to be an ambush predator (Argot 2004; but see Ercoli et al. 2012 and Janis et al. 2020 for alternative locomotive and dietary interpretations). However, sparassodonts were already declining in the Huayquerian SALMA (~8.50–5.17 Ma), and Thylacosmilus (alongside the last sparassodonts) make their last appearance in the “middle” Pliocene (Chapadmalan SALMA; 3.74–3.04 Ma). This means that there is at least a ~1 Ma gap between the extinction of Thylacosmilus and the appearance of large hypercarnivore felids like Smilodon in the fossil record, a gap which is unlikely to be just a taphonomic and/or sampling bias (Prevosti and Forasiepi 2018). An alternative but not exclusive explanation for the aliform expansion of the radius and the extremely developed collateral ligaments in the digits is that these structures could be related to swerving and dodging due to intraspecific behaviours (e.g., competition and displays during mating season).
Considering that Micrauchenia saladensis is a terrestrial mammal that was preserved in marine rocks (see Paleoenvironmental conditions of Bahía Inglesa Formation section), we cannot directly infer the environment in which this macraucheniid was living from the rock unit in which it was found. However, vegetation modelling for the continental western side of southern South America during the late Miocene suggests the presence of a proto-Atacama Desert at 24–26°S and south of it, the presence of temperate xerophytic shrubland followed by sclerophyll woodland and shrubland (Pound et al. 2011, 2012). Paleosols further support the onset of the hyperaridity of the Atacama Desert during the late Miocene related to the formation of the Central Andean rain-shadow, although not as intense as during the late Pliocene and Pleistocene (Rech et al. 2019). Therefore, considering the latitude of the Bahía Salado area, it is likely that M. saladensis was living in a coastal environment with sclerophyll woodland and shrubland vegetation (Fig. 22).
Body mass
It is difficult to estimate the body mass for Micrauchenia saladensis using the known limited fossil material combined with the unique anatomy of macraucheniids, of which no extant mammals are clear analogues. For example, if we estimate body mass of M. saladensis (SGO.PV.21700) and Macrauchenia patachonica (MACN PV 2) using the "all ungulates" radius R2 (log [body mass] = 2.7991 * log[R2] + 0.0890) and R4 (log[body mass] = 2.7703 * log[R4] – 0.0067) equations from Scott (1990); and we calculate the geometric mean of the estimates, we obtain 17.94 kg and 167.19 kg, respectively. These are both lower estimates than would be expected considering the overall dimensions of the different elements of the skeleton of both taxa (Tables S1-S6 in Online Resource 1), and that previous body mass estimations of Macrauchenia patachonica which vary from 830 kg (Fariña et al. 1998) to 1100 kg (Fariña 1996). These previous estimations for Macrauchenia were based on 66 craniodental and postcranial equations, and the scaling up and averaging of modern South American camelids (considered “morphological analogues” by Fariña 1996) respectively. If we use the ratio of the mass estimates between Macrauchenia patachonica and M. saladensis (i.e., 167.19/17.94 kg) and consider an estimation of 955.51 kg body mass for Macrauchenia patachonica (based on the geometric mean of the two previous estimations of Fariña 1996 and Fariña et al. 1998), M. saladensis would have had a body mass of around 102.52 kg. This is simply an estimate based on proportionality of two different body mass estimations, but it is consistent with previous body mass estimates for Theosodon garretorum and Theosodon lydekkeri of 158.04 kg and 130.94 kg, respectively, using craniodental equations (Cassini et al. 2012). These taxa were moderately but consistently bigger than M. saladensis considering linear measurements. However, even these and previous estimations have to be taken with caution as there is a considerable variation in body mass estimations depending in the method and equation used (McGrath et al. 2018), which is related to the unusual anatomy of macraucheniids when compared with the modern taxa used to generate these equations.
A different method for estimating body mass that is considered to be less affected by the potentially odd proportions of extinct taxa, is using occipital condyle width (OCW) regression equations (Engelman 2022). Micrauchenia (SGO.PV.21700) did not preserved a cranium, so measuring OCW directly is not possible. However, the interior width between the occipital facets of the atlas (i.e., 46 mm) can provide an estimation for OCW as the occipital condyles sit there. Using the regression equation for ungulates (ln(body mass) = 7.6451 × ln(OCW)2/3−8.0565), that included members of Artiodactyla, Perissodactyla, and Hyracoidea (Engelman 2022), we estimated a value of 42.35 kg for Micrauchenia. Using the same equation in other macraucheniids, we estimated the following body masses: Cramauchenia normalis (MPEF-PV 2524, OCW: 49.29 mm): 53.00 kg; Huayqueriana cf. H. cristata (IANIGLA-PV 29: 64.45 mm): 125.11 kg; Scalabrinitherium bravardi (MACN PV 13,082: 66.77 mm): 139.92 kg; Promacrauchenia antiquua (MACN PV 7986, OCW: 72.12 mm): 178.36 kg; and Macrauchenia patachonica (MNHN.F.PAM69, OCW: 107.85 mm; MACN PV 2, OCW: 106.27 mm): 591.79–619.03 kg. These estimates are considerably lower than previous body mass estimations for Huayqueriana (154.39–721.18 kg; Forasiepi et al. 2016) and Macrauchenia (830–1100 kg; Fariña 1996; Fariña et al. 1998), although we consider them plausible taking into consideration the bizarre anatomy of macraucheniids.
Macraucheniid diversity in the late Miocene
The presence of Micrauchenia saladensis in Bahía Salado increases the species richness and the diversity of body sizes of macraucheniids in the late Miocene, in particular of the Huayquerian SALMA (~8.50–5.17 Ma). Apart from M. saladensis, seven other macraucheniids species have been recognized for the late Miocene (i.e., Cullinia levis, Huayqueriana cristata, Paranauchenia hystata, Paranauchenia denticulata, Oxyodontherium zeballosi, Scalabrinitherium bravardi, and Promacrauchenia calchaquiorum). These are mostly medium-sized macraucheniids, except the small and gracile Cullinia levis (Fig. 1). Five of these (i.e., Huayqueriana cristata, Paranauchenia denticulata, Oxyodontherium zeballosi, Scalabrinitherium bravardi, and Promacrauchenia calchaquiorum) have been found within the Huayquerian SALMA.
The Huayquerian SALMA has been described as the highest diversity peak in macraucheniid evolution (McGrath et al. 2020), and after this period, macraucheniids of small size disappear from the fossil record to never return. By the late Pleistocene, some macraucheniids reached enormous sizes like the 592–1100 kg Macrauchenia patachonica (Fariña 1996; Fariña et al. 1998), Xenorhinotherium bahiense inferred to be of a similar body size as M. patachonica considering its dimensions (Tables S2-S6 in Online Resource 1), and the colossal 1200 kg Macraucheniopsis ensenadensis (Vizcaíno et al. 2012, based on geometric similarity of a 988 kg estimation for Macrauchenia patachonica), which were part of the South American megafauna. It is unclear why the small bodied macraucheniids disappeared. A hypothesis on the disappearance of the smaller macraucheniids like M. saladensis and Cullinia levis could be competition with the North American ungulates (i.e., equids, cervids, tayassuids, tapiroids) crossing into South America by the late Pliocene during the GABI. However, there was most likely a considerable gap between both events as these North American potential competitors are most likely absent from South America by most authors during the late Miocene (Woodburne 2010; Goin et al. 2012; Cione et al. 2015; Gasparini et al. 2021). Even though some authors claim an earlier date for the GABI based on ungulates with North American affinities in supposed late Miocene deposits of Peruvian Amazon (Campbell et al. 2000; Frailey and Campbell 2012; Prothero et al. 2014), these have been recently questioned in terms of their taxonomy and provenance, being most likely Quaternary remains (Gasparini et al. 2021).
A more likely possibility that is consistent with the available evidence is that this particular disappearance of small macraucheniid species was a stochastic process within a general trend of reduction of diversity in macraucheniids. Indeed, beginning in the late Miocene, litopterns (i.e., macraucheniids and proterotheriids), and notoungulates started experiencing a steep reduction in diversity (i.e., number of genera; Marshall and Cifelli 1990; Bond et al. 1995; Croft et al. 2020), and therefore just by chance, the small forms of macraucheniids could have disappeared. There are currently no clear reasons for this general decline in diversity after the late Miocene and towards the Pliocene/Pleistocene, but it could be potentially related to the global cooling and climatic instability triggered by expansions of ice sheets in west-Antarctica and in the Arctic (Zachos et al. 2001) that, coupled with the uplift of the Andes creating rain shadow effects, led to an increase aridification and woodland retreat (Pascual et al. 1985; Pascual and Jaureguizar 1990; Rech et al. 2019). These environmental changes have been also argued as one of the potential causes for the sparassodont demise in the “middle” Pliocene (Chapadmalan SALMA; 3.74–3.04 Ma; Prevosti et al. 2013).
Phylogenetic considerations
The results of our phylogenetic analyses are consistent between the different methods here used, recovering similar positions for most of the taxa included in the phylogeny, although with a degree of uncertainty in particular regarding the affinities of the members of the subfamily Macraucheniinae. The newly described Micrauchenia saladensis, even though it always recovered within Macraucheniinae, varies from more basal in the tip-dated Bayesian and parsimony majority rule consensus trees, to more derived in the undated Bayesian consensus tree. These results reflect the degree of uncertainty of the phylogenetic placement of M. saladensis within Macraucheniinae, and can be attributed to the lack of craniodental characters scored for this taxon, and also the lack of postcranial information for most of the macraucheniids included. Indeed, the absence of detailed descriptions of the postcranial elements of macraucheniids, apart from Theosodon and Macrauchenia, make comparisons difficult. Even though there seems to be a striking overall anatomical uniformity of postcranial characters between earlier macraucheniines (late Miocene-Pliocene) and later Pleistocene species like Macrauchenia patachonica as previous authors have noted (e.g., Parodi 1931), our postcranial descriptions of M. saladensis and comparisons with members of Macraucheniinae show that there are postcranial features that have potential to help untangle the phylogenetic affinities within this subfamily, and also the relationships of earlier macraucheniids. Here we included eight postcranial characters, although as more postcranial elements are found (either in the field or museum collections) and described, these could be potentially refined by dividing into more states, and new characters could be added, such as those that encapsulate vertebral elements, for example. This is important, in light of the fact that some of the dental characters employed here and in previous studies (Schmidt and Ferrero 2014; Forasiepi et al. 2016; McGrath et al. 2018) are prone to be affected by the level of tooth wear as macraucheniids increased their level of hypsodonty. Characters like the length of m2 (char. 6), the parastyle projection on P3-P4 (char. 17), position of the hypocone on M2 relative to the protocone (char. 20) and the relative position of the metaconid on m2 (char. 26) could be affected depending on degree of wear (Lobo 2015). This is problematic because the level of tooth wear tends to give more information about age and diet, instead of phylogenetic affinities.
Our maximum parsimony results broadly agree with previous parsimony studies using only craniodental characters (Schmidt and Ferrero 2014; Forasiepi et al. 2016; McGrath et al. 2018), and when M. saladensis is removed from the consensus, we had a comparable degree of resolution (Online Resource 5 Fig. S2). In terms of the synapomorphies for the subfamily Macraucheniinae, our results are in general consistent with previous studies (Schmidt and Ferrero 2014; McGrath et al. 2018), although we found eight unambiguous synapomorphies, instead of five and six, respectively. The new synapomorphies were: (a) the premaxillae and maxillae are sutured sagitally in dorsal view (char. 8) and (b) the coronal plane passing through infraorbital foramen is anterior to M3 (char 9). We did not find mesodont dentition (char. 15) as an unambiguous synapomorphy for Macraucheniinae as in previous studies (Schmidt and Ferrero 2014; McGrath et al. 2018), although it is likely to be so, as this feature has not been found in early macraucheniids or “cramaucheniids” which are all brachyodont (i.e., HI < 1.45). The fusion of the ulna and radius is probably an additional synapomorphy for the subfamily Macraucheniinae considering that all late Miocene to late Pleistocene adult macraucheniids found so far possess this feature (Soria 2001). However, this feature was not found as an unambiguous synapomorphy in our analysis probably because of missing data, as most macraucheniids species are only known from craniodental remains.
Anaya and MacFadden (1995) assigned the macraucheniid material from Inchasi beds to Promacrauchenia sp. based on a clear size difference with the Pleistocene Macrauchenia and the presence of Promacrauchenia in other Pliocene localities. Contrary to this interpretation, the phylogenetic position of Promacrauchenia sp. (Anaya and MacFadden 1995) recovered in the Bayesian and parsimony phylogenetic analyses, strongly suggests that this is a different taxon from Promacrauchenia calchaquiorum and Promacrauchenia antiquua (Promacrauchenia spp.). Among the five unambiguous synapomorphies recovered from the parsimony-based majority rule consensus tree (Fig. 20b) that link Promacrauchenia sp. (Anaya and MacFadden 1995) with Macrauchenia patachonica and Macraucheniopsis ensenadensis, three of them are preserved in the Promacrauchenia sp. (Anaya and MacFadden 1995) specimens and are absent in the Promacrauchenia spp.: (a) the absence of an entoconid on m1-m2 (char. 3); (b) a strongly compressed trigonid of m1 against the talonid of p4 (char. 4); and (e) the absence of an entolophid on m1-m2 (char. 23). Indeed, the absence of entoconid and entolophid in the lower molar series of the macraucheniines from Inchasi was previously observed by Schmidt (2013), although the phylogenetic implications were never empirically tested until now. Apart from these dental features, there are postcranial characteristics that are not consistent with what is known from Promacrauchenia. One of the diagnostic features of the genus Promacrauchenia is that the width at the level of the distal articular surfaces of the ulna-radius is less than the width at the level of the aliform expansion (Pascual et al. 1966). Promacrauchenia sp. (Anaya and MacFadden 1995) shows the opposite condition, exhibiting a wider aliform expansion than the distal end of the ulna-radius, which is more similar to Macrauchenia and M. saladensis. However, a feature that clearly distinguishes Promacrauchenia sp. (Anaya and MacFadden 1995) from the latter two genera is that the aliform expansion is situated in a relatively more proximal position of the radius-ulna (Schmidt 2013). Taking all this evidence together in terms of size, dental and postcranial features, the macraucheniine specimens from Inchasi beds are probably a new and distinctive genus/species of macraucheniid, which merits a new in-detail taxonomic revision.
Although we could not resolve the specific position of M. saladensis within Macraucheniinae, there are distinct anatomical features that allow us to confidently assign it to a new genus/species. Its size is particularly small for a macraucheniid from the late Miocene, being of a similar size of earlier macraucheniids like Cramauchenia normalis and Coniopternium andinum. The type specimen is not a juvenile, as it exhibits a complete fusion of the ulna and radius, and completely lacks epiphyseal plates in the distal end of the ulna and radius and in the metacarpals and phalanges (Fig. 9b, c). Apart from the clear size difference, the relative level of development of its ulna-radius aliform expansion and position is different from Promacrauchenia spp. and Promacrauchenia sp. (Anaya and MacFadden 1995), being wider that the ulna-radius distal end (unlike Promacrauchenia spp.) and distally located (unlike Promacrauchenia sp.; Anaya and MacFadden 1995). Unfortunately, not much is known about the postcranium of the earlier members of Macraucheniinae, but the smallest member of this subfamily so far described in terms of the dental and postcranial remains is Cullinia levis (Cabrera and Kraglievich 1931; Bond and López 1995). However, there are two main anatomical features that allow us to discard Cullinia levis as the species here described. First, Cullinia levis is consistently larger than M. saladensis. In the case of the pisiform, in which a direct comparison is possible, Cullinia cf. C. levis (MLP 55-IV-28–97) is ~40% larger than M. saladensis (SGO.PV.21700; Fig. 14a-f, i, j and Table S4 in Online Resource 1). In addition, the size of Cullinia levis considering dental and postcranial remains is larger than Coniopternium andinum (Schmidt 2013; McGrath et al. 2020). In contrast, M. saladensis tends to be slightly smaller than Coniopternium andinum considering linear measurements of its digits (Table S6 in Online Resource 1). Second, one of the diagnostic features of Cullinia levis is that it is a very gracile macraucheniid, presenting metapodials that are proportionally longer than Theosodon or later macraucheniines (Cabrera and Kraglievich 1931; Pascual et al. 1966; Bond and López 1995). This feature can be clearly observed in the thin and elongated Mt II and Mt IV of Cullinia levis (MLP 71-IX-2–5; MLP 29-IX-1–78; Fig. S3e, g in Online Resource 5). The relative length of the metapodials of M. saladensis is similar to Theosodon spp., and within the range observed in our sample of macraucheniids (Table S5 in Online Resource 1), contrary to what would be expected if these were remains of Cullinia levis (Table S7 in Online Resource 1 and Fig. S3 in Online Resource 5). The metapodials of Cullinia levis (i.e., Mt II and Mt IV) are always proportionally mediolaterally thinner than the metapodials of Theosodon spp. (i.e., considering total length). Other macraucheniids from the late Miocene from which not many postcranial materials are available or described, such as Scalabrinitherium bravardi, Paranauchenia denticulata or Oxyodontherium zeballosi, are just too large to be the SGO.PV.21700 materials considering that they are consistently larger than Cullinia levis when their dentition is compared (McGrath et al. 2020). Taking all of this evidence together, we are confident that our taxonomic assignment of SGO.PV.21700 to the new species Micrauchenia saladensis is justified.
The description of M. saladensis provides important data on the postcranial anatomy of macraucheniids. The postcranial anatomy of the Santacrucian SALMA (early Miocene) specimens of Theosodon and the late Pleistocene Macrauchenia and Xenorhinotherium is well known, but not much is known (or described) about macraucheniid postcranial anatomy during the temporal gap between these two intervals. From anatomical comparisons between different macraucheniids, it can be argued that even though the general bauplan in macraucheniids is very conserved, there are clear anatomical trends in macraucheniid macroevolution: the retraction and atrophy of the nasal bones, nostrils becoming more dorsally positioned, increasing tooth crown height, the fusion of the ulna and radius, the fusion of the tibia and fibula, the development of the aliform expansion of the radius, among other features, some of which we identified as unambiguous synapomorphies in our parsimony analyses. These features appear to be ubiquitous and developed to a certain degree in all the macraucheniids found so far from the late Miocene to the late Pleistocene/Holocene, and are clearly absent in earlier macraucheniids.
Conclusions
We describe a new macraucheniine macraucheniid Micrauchenia saladensis (SGO.PV.217000) from the late Miocene (Huayquerian SALMA), the first litoptern and SANU from the marine Bahía Inglesa Formation, Chile. With an estimated body mass of 53–102.52 kg, M. saladensis is also the smallest macraucheniine yet described, increasing the diversity of macraucheniid body sizes for the Huayquerian SALMA. The location of this specimen within the Bahía Inglesa Formation is curious, as the postcranial anatomy of M. saladensis is consistent with a terrestrial animal, likely one with a subunguligrade stance and a forelimb adapted for cursorial locomotion, as is seen in other macraucheniids. This suggests an allochthonous position for Micrauchenia, which probably died near the Bahía Salado coast, and was subsequently swept away by the tide before being deposited.
M. saladensis presents features that have been observed only in the subfamily Macraucheniinae, such as the fusion of the ulna and radius and the presence of a radial aliform expansion (Soria 1981). We consider the fusion of the ulna and radius as a cursorial specialization for restricting pronation-supination movement, favouring parasagittal motion. The radial aliform expansion provided a large area for the insertion of an enlarged deep digital flexor muscle which would have likely facilitated strong flexion of the forefoot and withstood higher transverse stresses (Fariña et al. 2005). We suggest that this unusual osteological feature and its related muscular development was used for rapid flexion of the manus and aided in sudden movements, such as swerving and dodging, either to avoid predators, or for intraspecific behaviours (e.g., competition, mating).
Our phylogenetic analyses corroborate M. saladensis as member of subfamily Macraucheniinae, although with uncertain affinities within this subfamily. This is likely due to the lack of diagnostic craniodental remains in Micrauchenia, and also the lack of postcranial remains associated to most macraucheniid species. Indeed, apart from Theosodon and Macrauchenia, the postcrania of most other macraucheniids are insufficiently known. In that sense, Micrauchenia provides relevant anatomical information in macraucheniid evolution.
Data availability
All data generated or analysed during this study are included in this published article as Online Resources 1-5. In addition, the matrix for the phylogenetic analysis is available online on Morphobank (morphobank.org) under the project number 3933.
References
Achurra LE (2004) Cambios del nivel del mar y evolución tectónica de la cuenca neógena de Caldera, III Región. Dissertation, Universidad de Chile
Ameghino F (1883) Sobre una colección de mamíferos fósiles del piso Mesopotámico de la formación Patagónica recogidos en las barrancas del Paraná por el profesor Pedro Scalabrini. Bol Acad Nac Cienc Córdoba 5:101–116
Ameghino F (1887) Enumeración sistemática de las especies de mamíferos fósiles coleccionados por Carlos Ameghino en los terrenos Eocenos de la Patagonia Austral y depositados en el Museo de La Plata. Bol Mus La Plata 1:1–26
Ameghino F (1889) Contribución al conocimiento de los mamíferos fósiles de la República Argentina. Actas Acad Nac Cienc Córdoba 6:1–1027
Ameghino F (1894a) Sur les oiseaux fossiles de Patagonie et la faune mammalogique des couches à Pyrotherium. Bol Inst Geogr Argent 15:501–660
Ameghino F (1894b) Sur les ongulés fossiles de l’Argentine. Rev Jard Zoo Buenos Ayres Tomo II:193–303
Ameghino F (1897) Mammiféres crétacés de l’Argentine (Deuxième contribution à la connaissance de la fauna mammalogique de couches à Pyrotherium). Bol Inst Geogr Argent 18:406–521
Ameghino F (1902) Première contribution à la connaissance de la faune mammalogique des couches à Colpodon. Bol Acad Nac Cienc Córdoba 17:71–138
Ameghino F (1904) Nuevas especies de mamíferos cretáceos y terciarios de la República Argentina. An Soc Cient Argent 56–58:1–142
Anaya F, MacFadden BJ (1995) Pliocene Mammals from Inchasi, Bolivia: the endemic fauna just before the great American interchange. Bull Fla Mus Nat Hist 39:87–140
Antoine PO, Marivaux L, Croft DA, et al (2012) Middle Eocene rodents from Peruvian Amazonia reveal the pattern and timing of caviomorph origins and biogeography. Proc R Soc B Biol Sci 279:1319–1326. https://doi.org/10.1098/rspb.2011.1732
Aramayo SA, de Bianco TM (2009) Late Quaternary palaeoichnological sites from the Southern Atlantic coast of Buenos Aires Province, Argentina: mammal, bird and hominid evidence. Ichnos 16:25–32. https://doi.org/10.1080/10420940802470714
Aramayo SA, de Bianco TM, Bastianelli N V, Melchor RN (2015) Pehuen Co: Updated taxonomic review of a late Pleistocene ichnological site in Argentina. Palaeogeogr Palaeoclimatol Palaeoecol 439:144–165. https://doi.org/10.1016/j.palaeo.2015.07.006
Argot C (2001) Functional-adaptive anatomy of the forelimb in the Didelphidae, and the paleobiology of the Paleocene marsupials Mayulestes ferox and Pucadelphys andinus. J Morphol 247:51–79. https://doi.org/10.1002/1097-4687(200101)247:1<51::aid-jmor1003>3.0.co;2-%23
Argot C (2004) Functional-adaptive features and palaeobiologic implications of the postcranial skeleton of the late Miocene sabretooth borhyaenoid Thylacosmilus atrox (Metatheria). Alcheringa 28:229–266. https://doi.org/10.1080/03115510408619283
Argot C (2013) Postcranial analysis of a carnivoran-like archaic ungulate: the case of Arctocyon primaevus (Arctocyonidae, Mammalia) from the Late Paleocene of France. J Mamm Evol 20:83–114. https://doi.org/10.1007/s10914-012-9198-x
Aurich C, Bragulla H, Budras K-D, et al (2020) Veterinary Anatomy of Domestic Animals, 7th edn. Georg Thieme Verlag, Stuttgart · New York
Bastos ACF, Bergqvist LP (2007) A postura locomotora de Protolipterna ellipsodontoides Cifelli, 1983 (Mammalia: Litopterna: Protolipternidae) da Bacia de São José de Itaboraí, Rio de Janeiro (Paleoceno superior). Anu Inst Geociênc 30:58–66
Beilinson E, Gasparini GM, Tomassini RL, et al (2017) The Quequén Salado river basin: Geology and biochronostratigraphy of the Mio-Pliocene boundary in the southern Pampean plain, Argentina. J S Am Earth Sci 76:362–374. https://doi.org/10.1016/j.jsames.2017.04.002
Bergqvist LP (2008) Postcranial skeleton of the upper Paleocene of Itaboraí Basin, Brazil. In: Sargis EJ, Dagosto M (eds) Mammalian Evolutionary Morphology: A tribute to Frederik S. Szalay. Springer-Verlag, New York, pp 107–133
Blanco RE, Jones WW, Yorio L, Rinderknecht A (2021) Macrauchenia patachonica Owen, 1838: Limb bones morphology, locomotory biomechanics, and paleobiological inferences. Geobios 68:61–70. https://doi.org/10.1016/j.geobios.2021.04.006
Bonaparte JF, Morales J (1997) Un primitivo Notonychopidae (Litopterna) del Paleoceno inferior de Punta Peligro, Chubut, Argentina. Estud Geol 274:263–274. https://doi.org/10.3989/egeol.97535-6232
Bond M (1999) Quaternary native ungulates of southern South America. A synthesis. In: Rabassa J, Salemme M (eds) Quaternary of South America and Antarctic Península, 12. Rotterdam, pp 177–205
Bond M, Cerdeño E, Lopez G (1995) Los ungulados nativos de América del Sur. In: Alberdi M, Leone G, Tonni E (eds) Evolución Biológica y Climática de la Región Pampeana Durante los Últimos Cinco Millones de Años. Un Ensayo de Correlación con el Mediterráeno Occidental. Museo Nacional de Ciencias Naturales, Madrid, pp 260–275
Bond M, López GM (1995) Los Macraucheniidae (Mammalia, Litopterna) de la Formación Arroyo Chasicó (Partido de Vallarino, Pcia. de Buenos Aires). In: Actas, IV Jornadas Geológicas y Geofísicas Bonaerenses, Junín, Argentina, pp 23–27
Bond M, Reguero MA, Vizcaíno SF, Marenssi SA (2006) A new ‘South American ungulate’ (Mammalia: Litopterna) from the Eocene of the Antarctic Peninsula. Geol Soc Lond Spec Publ 258:163–176. https://doi.org/10.1144/GSL.SP.2006.258.01.12
Bond M, Tejedor MF, Campbell KE, et al (2015) Eocene primates of South America and the African origins of New World monkeys. Nature 520:538–541. https://doi.org/10.1038/nature14120
Buckley M (2015) Ancient collagen reveals evolutionary history of the endemic South American ‘ungulates.’ Proc R Soc B Biol Sci 282:20142671. https://doi.org/10.1098/rspb.2014.2671
Burmeister G (1864a) Beschreibung der Macrauchenia patachonica Owen (Opisthorhinus falkoneri Brav.) nach A. Bravard’s Zeichnungen und den im Museo zu Buenos Aires vorhandenen Resten entworfen. Abhandl Naturf Ges Halle 1:75–112.
Burmeister G (1864b) Descripción de la Macrauchenia patachonica. In: Anales del Museo Público de Buenos Aires para dar a conocer los objetos de historia natural nuevos o poco conocidos conservados en este establecimiento. Tomo I. Imprenta de "La Tribuna", Buenos Aires, pp 32–66
Cabrera Á, Kraglievich L (1931) Diagnosis previas de los ungulados fósiles del Arroyo Chasicó. N Prelim Mus La Plata 1:107–113
Campbell B (1936) The comparative myology of the forelimb of the hippopotamus, pig and tapir. Am J Anat 59:201–247. https://doi.org/10.1002/aja.1000590203
Campbell KEJ, Frailey CD, Pittman LR, Romero-Pittman L (2000) The late Miocene gomphothere Amahuacatherium peruvium (Proboscidea: Gomphotheriidae) from Amazonian Peru: implications for the Great American Faunal Interchange. Bol Estud Reg Inst Geol Min Met 23:1–152
Carrano MT (1997) Morphological indicators of foot posture in mammals: A statistical and biomechanical analysis. Zool J Linn Soc 121:77–104. https://doi.org/10.1111/j.1096-3642.1997.tb00148.x
Carreño C (2012) Ambiente deposicional de la Formación Bahía Inglesa (Neógeno) en la Cuenca de Caldera, III Región, Chile. Dissertation, Universidad de Chile
Cartelle C, Lessa G (1988) Descrição de um gênero e espécie de Macraucheniidae (Mammalia, Litopterna) do Pleistoceno final do Brasil. Paula-Coutiana 3:3–26
Cassini GH, Cerdeño E, Villafañe AL, Muñoz NA (2012) Paleobiology of Santacrucian native ungulates (Meridiungulata: Astrapotheria, Litopterna and Notoungulata). In: Vizcaíno SF, Kay RF, Bargo MS (eds) Early Miocene Paleobiology in Patagonia: High-Latitude Paleocommunities of the Santa Cruz Formation. Cambridge University Press, Cambridge, pp 243–286
Cerdeño E, Chiesa J, Ojeda G (2008) Presence of Oxyodontherium (Macraucheniidae, Litopterna) in the Río Quinto Formation, San Luis (Argentina). J S Am Earth Sci 25:217–226. https://doi.org/10.1016/j.jsames.2007.06.004
Cerdeño E, Vera B (2015) A new Leontiniidae (Notoungulata) from the Late Oligocene beds of Mendoza Province, Argentina. J Syst Palaeontol 13:943–962. https://doi.org/10.1080/14772019.2014.982727
Cifelli R (1983a) The origin and affinities of the South American Condylarthra and early Tertiary Litopterna (Mammalia). Am Mus Novit 2772:1–49
Cifelli RL (1983b) Eutherian tarsals from the late Paleocene of Brazil. Am Mus Novit 2761:1–31
Cifelli RL (1985) South American ungulate evolution and extinction. In: Stehli FG, Webb SD (eds) The Great American Biotic Interchange. Springer US, pp 249–266
Cifelli RL (1993) The phylogeny of the native South American ungulates. In: Szalay FS, Novacek MJ, Mckenna MC (eds) Mammal Phylogeny: Placentals. Springer-Verlag, New York, pp 195–216
Cifelli RL, Guerrero Díaz J (1997) Litopterns. In: Kay RF, Madden RH, Cifelli RL, Flynn JJ (eds) Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Smithsonian Institution Press, Washington, D.C., pp 289–302
Cifelli RL, Soria MF (1983) Systematics of the Adianthidae (Litopterna , Mammalia). Am Mus Novit 2771:1–25
Cione AL, Gasparini GM, Soibelzon E (2015) The Great American Biotic Interchange: a South American Perspective. Springer, Dordrecht
Constantinescu GM, Habel RE, Hillebrand A, et al (2018) Illustrated Veterinary Anatomical Nomenclature, 4th edn. Georg Thieme Verlag, Stuttgart
Cozzuol MA (2006) The Acre vertebrate fauna: Age, diversity, and geography. J S Am Earth Sci 21:185–203. https://doi.org/10.1016/j.jsames.2006.03.005
Croft DA, Flynn JJ, Wyss AR (2004) Notoungulata and Litopterna of the Early Miocene Chucal fauna, northern Chile. Fieldiana: Geology 50:1–52. https://doi.org/10.5962/bhl.title.5228
Croft DA, Gelfo JN, López GM (2020) Splendid Innovation: The Extinct South American Native Ungulates. Annu Rev Earth Planet Sci 48:11.1–11.32. https://doi.org/10.1146/annurev-earth-072619-060126
Cuitiño JI, Gutstein CS, Ugalde R, Muñoz F (2021) Variaciones de paleoambientes marinos de alta bioproductividad: el caso de la Formación Bahía Inglesa, Neógeno de Atacama, Chile. In: Actas XVII Reunión Argentina de Sedimentología. Paraná, Argentina, p 131
Delfino M, Piras P, Smith T (2005) Anatomy and phylogeny of the gavialoid crocodylian Eosuchus lerichei from the Paleocene of Europe. Acta Palaeontol Pol 50:565–580
Delignette-Muller ML, Dutang C (2004) fitdistrplus: an R package for fitting distributions. J Stat Softw 64:1–34
Denoix J-M (2019) Essentials in Clinical Anatomy of the Equine Locomotor System, 1st edn. Taylor & Francis Group
Deschamps CM, Vucetich MG, Montalvo CI, Zárate MA (2013) Capybaras (Rodentia, Hydrochoeridae, Hydrochoerinae) and their bearing in the calibration of the late Miocene-Pliocene sequences of South America. J S Am Earth Sci 48:145–158. https://doi.org/10.1016/j.jsames.2013.09.007
Dozo MT, Vera B (2010) First skull and associated postcranial bones of Macraucheniidae (Mammalia, Litopterna) from the Deseadan SALMA (Late Oligocene) of Cabeza Blanca (Chubut, Argentina). J Vertebr Paleontol 30:1818–1826. https://doi.org/10.1080/02724634.2010.521534
Engelman RK (2022) Occipital condyle width (OCW) is a highly accurate predictor of body mass in therian mammals. BMC Biol 20:37:1–44
Ercoli MD, Prevosti FJ, Álvarez A (2012) Form and function within a phylogenetic framework: Locomotory habits of extant predators and some Miocene Sparassodonta (Metatheria). Zool J Linn Soc 165:224–251. https://doi.org/10.1111/j.1096-3642.2011.00793.x
Etienne C, Houssaye A, Hutchinson JR (2021) Limb myology and muscle architecture of the Indian rhinoceros Rhinoceros unicornis and the white rhinoceros Ceratotherium simum (Mammalia: Rhinocerotidae). PeerJ 9:e11314. https://doi.org/10.7717/peerj.11314
Evans H, Lahunta A de (2012) Miller’s Anatomy of the Dog, 4th Ed. Saunders, Philadelphia
Fariña RA (1996) Trophic relationships among Lujanian mammals. Evol Theory 11:125–134
Fariña RA, Blanco RE, Christiansen P (2005) Swerving as the escape strategy of Macrauchenia patachonica Owen (Mammalia; Litopterna). Ameghiniana 42:751–760
Fariña RA, Vizcaíno SF, Bargo MS (1998) Body mass estimation in Lujanian (Late Pleistocene-Early Holocene of South America) mammal megafauna Pleistocene megafauna. Mastozool Neotrop 5:87–108
Forasiepi AM, Macphee RDE, Hernández del Pino S, et al (2016) Exceptional skull of Huayqueriana (Mammalia, Litopterna, Macraucheniidae) from the late Miocene of Argentina: anatomy, systematics, and paleobiological implications. Bull Am Mus Nat Hist 404:1–76
Frailey CD, Campbell KE (2012) Two new genera of peccaries (Mammalia, Artiodactyla, Tayassuidae) from upper Miocene deposits of the Amazon Basin. J Paleontol 86:852–877. https://doi.org/10.1666/12-012.1
Gasparini GM, Dutra RP, Perini FA, et al (2021) On the supposed presence of Miocene Tayassuidae and Dromomerycinae (Mammalia, Cetartiodactyla) in South America. Am Mus Novit 3968:1–27. https://doi.org/10.1206/3968.1
Gelfo JN, López GM, Lorente M (2016) Los ungulados arcaicos de América del Sur: “Condylarthra” y Litopterna. Contrib Cient Mus Argent Cienc Nat “Bernardino Rivadavia" 6:285–291. https://doi.org/10.1017/CBO9781107415324.004
Gelfo JN, López GM, Santillana SN (2017) Eocene ungulate mammals from West Antarctica: Implications from their fossil record and a new species. Antarct Sci 29:445–455. https://doi.org/10.1017/S0954102017000244
Gervais MP (1855) Recherches sur les mammifères fossiles de l’Amérique Méridonale. In: Castelnau F de (ed) Animaux nouveaux ou rares recueillis pendant l’expedition dans les parties centrales de l’Amérique du Sudde Rio de Janeiro á Lima, et de Lima au Para. P. Bertrand, Libraire-Éditeur, Paris, pp 1–63
Godoy E, Marquardt C, Blanco N (2003) Carta Caldera, Región de Atacama. In: Serie Geología Básica. Servicio Nacional de Geología y Minería, Santiago, p 38
Goin FJ, Gelfo JN, Chornogubsky L, et al (2012) Origins, radiations, and distribution of South American mammals. In: Patterson BD, Costa LP (eds) Bones, Clones, and Biomes: The History and Geography of Recent Neotropical Mammals. The University of Chicago Press, Chicago, pp 20–50
Goloboff PA, Catalano SA (2016) TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 32:221–238. https://doi.org/10.1111/cla.12160
Grigg G, Kirshner D (2015) Biology and Evolution of Crocodylians. Cornell University Press, New York
Gunnell GF, Boyer DM, Friscia AR, et al (2018) Fossil lemurs from Egypt and Kenya suggest an African origin for Madagascar’s aye-aye. Nat Commun 9:1–12. https://doi.org/10.1038/s41467-018-05648-w
Gutstein CS, Cherem JJ, Cozzuol MA, Suárez M (2007) Primer registro de capibara (Hydrochoeridae, Rodentia) de Chile (Fm. Bahía Inglesa). Biol Res 40:R-128
Harrison LB, Larsson HCE (2015) Among-character rate variation distributions in phylogenetic analysis of discrete morphological characters. Syst Biol 64:307–324. https://doi.org/10.1093/sysbio/syu098
Henríquez AA (2006) Variaciones locales del nivel del mar en las cuencas neógenas de Caldera, III Región y Arauco, VIII Región: deducción de tasas de alzamiento y subsidencia tectónica. Dissertation, Universidad de Chile
Hildebrand M, Goslow GE (2001) Analysis of Vertebrate Structure, 5th Ed. John Wiley & Sons, Inc., New York
Holmgren N (1952) An embryological analysis of the mammalian carpus and its bearing upon the question of the origin of the tetrapod limb. Acta Zool 33:1–115
Hua S, Jouve S (2004) A primitive marine gavialoid from the Paleocene of Morocco. J Vertebr Paleontol 24:341–350. https://doi.org/10.1671/1104
Huxley T (1880) On the application of the laws of evolution to the arrangement of the Vertebrata, and more particularly of the Mammalia. Proc Zool Soc Lond 43:649–662
International Committee on Veterinary Gross Anatomical Nomenclature (2017) Nomina Anatomica Veterinaria, 6th edn. Editorial Committee Hanover (Germany), Ghent (Belgium), Columbia, MO (U.S.A.), Rio de Janeiro (Brazil)
Janis CM, Figueirido B, DeSantis L, Lautenschlager S (2020) An eye for a tooth: Thylacosmilus was not a marsupial “saber-tooth predator.” PeerJ 2020:1–36. https://doi.org/10.7717/peerj.9346
Ji Q, Luo ZX, Yuan CX, et al (2002) The earliest known eutherian mammal. Nature 416:816–822. https://doi.org/10.1038/416816a
Kneepkens AFLM, Badoux DM, Macdonald AA (1989) Descriptive and comparative myology of the forelimb of the babirusa (Babyrousa babyrussa L. 1758). Anat Histol Embryol 18:349–365. https://doi.org/10.1111/j.1439-0264.1995.tb00035.x
Köhler-Rollefson IU (1991) Camelus dromedarius. Mamm Species 375:1–8 https://doi.org/10.2307/3504297
Kraglievich L (1932) Nuevos apuntes para la geología y paleontología uruguayas. An Mus Hist Nat Montevideo 3:257–321
Kraus R (1998) The cranium of Piscogavialis jugaliperforatus n. gen., n. sp. (Gavialidae, Crocodylia) from the Miocene of Peru. PalZ 72:389–405. https://doi.org/10.1007/bf02988368
Le Roux JP, Achurra L, Henríquez Á, et al (2016) Oroclinal bending of the Juan Fernández Ridge suggested by geohistory analysis of the Bahía Inglesa Formation, north-central Chile. Sediment Geol 333:32–49. https://doi.org/10.1016/j.sedgeo.2015.12.003
Lessa GM (1992) Estudo descritivo de Xenorhinotherium bahiense Cartelle & Lessa, 1998 e comparação com outras espécies de Macraucheniidae (Litopterna, Mammalia). Dissertation, Universidade Federal do Rio de Janeiro
Lewis P (2001) A likelihood approach to estimating phylogeny from discrete morphological character data. Syst Biol 50:913–925. https://doi.org/10.1080/106351501753462876
Linnaeus C (1758) Systema Naturae per Regna Tria Naturae. 10th edn. Laurentii Salvii, Stockholm
Lobo LS (2015) Estudo da morfologia dentária de Xenorhinotheirum bahiense Cartelle & Lessa, 1988 (Litopterna, Macraucheniidae). Dissertation, Universidade Federal de Viçosa
Luo ZX, Yuan CX, Meng QJ, Ji Q (2011) A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 476:442–445. https://doi.org/10.1038/nature10291
MacFadden BJ, Anaya F, Argollo J (1993) Magnetic polarity stratigraphy of Inchasi: a Pliocene mammal-bearing locality from the Bolivian Andes deposited just before the Great American Interchange. Earth Planet Sci Lett 114:229–241. https://doi.org/10.1016/0012-821X(93)90027-7
MacLaren JA, McHorse BK (2020) Comparative forelimb myology and muscular architecture of a juvenile Malayan tapir (Tapirus indicus). J Anat 236:85–97. https://doi.org/10.1111/joa.13087
Makhdoomi DM, Gazi MA, Ul Nabi S, Ahmed S (2013) Morphometric studies on adult double humped camel of Ladakh, India. Emirates J Food Agric 25:544–548. https://doi.org/10.9755/ejfa.v25i7.15999
Manzuetti A, Perea D, Ubilla M, Rinderknecht A (2018) First record of Smilodon fatalis Leidy, 1868 (Felidae, Machairodontinae) in the extra-Andean region of South America (late Pleistocene, Sopas Formation), Uruguay: Taxonomic and paleobiogeographic implications. Quat Sci Rev 180:57–62. https://doi.org/10.1016/j.quascirev.2017.11.024
Marquardt C (1999) Neotectónica de la franja costera y aportes a la geología regional entre Caldera y Caleta Pajonal (27°–27°4´), III Región de Atacama. Dissertation, Universidad de Chile
Marquardt C, Blanco N, Godoy E, et al (2000) Estratigrafía del Cenozoico Superior en el área de Caldera (26°45′28″S), III Región de Atacama, Chile. In: IX Congreso Geológico Chileno. Puerto Varas, Chile, pp 504–508
Marshall LG, Cifelli RL (1990) Analysis of changing diversity patterns in Cenozoic land mammal age faunas, South America. Palaeovertebrata 19:169–210
Matthew WD (1915) A revision of the lower Eocene Wasatch and Wind River faunas. Part II. Order Condylarthra, Family Hyopsodontidae. Bull Am Mus Nat Hist 9:311–328
McGrath AJ, Anaya F, Croft DA (2018) Two new macraucheniids (Mammalia: Litopterna) from the late middle Miocene (Laventan South American Land Mammal Age) of Quebrada Honda, Bolivia. J Vertebr Paleontol 38:e1461632. https://doi.org/10.1080/02724634.2018.1461632
McGrath AJ, Anaya F, Croft DA (2020) New proterotheriids from the middle Miocene of Quebrada Honda, Bolivia, and body size and diversity trends in proterotheriid and macraucheniid litopterns (Mammalia). Ameghiniana 57:159–188. https://doi.org/10.5710/AMGH.03.03.2020.3268
McHorse BK, Biewener AA, Pierce SE (2019) The evolution of a single toe in horses: causes, consequences, and the way forward. Integr Comp Biol 59:638–655
McKenna MC (1975) Toward a phylogenetic classification of the Mammalia. In: Luckett WP, Szalay FS (eds) Phylogeny of the Primates. Plenum Press, New York, pp 21–46
Miller KG, Kominz A, Browning J V, et al (2005) The Phanerozoic record of sea level change. Science 310:1293–1298
Novacek MJ (1986) The primitive eutherian dental formula. J Vertebr Paleontol 6:191–196
O’Dea A, Lessios HA, Coates AG, et al (2016) Formation of the Isthmus of Panama. Sci Adv 2:e1600883. https://doi.org/10.1126/sciadv.1600883
O’Leary MA, Bloch JI, Flynn JJ, et al (2013) The placental mammal ancestor and the Post-K-Pg radiation of placentals. Science 339:662–667. https://doi.org/10.1126/science.1229237
Owen R (1838) Part 1: Fossil Mammalia. In: Darwin CR (ed) The Zoology of the Voyage of H. M. S. Beagle, Under the Command of Captain Fitz Roy During the Years 1832 to 1836. Smith, Elder and Co, London, pp 1–111
Paradis E, Claude J, Strimmer K (2004) APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20:289–290. https://doi.org/10.1093/bioinformatics/btg412
Parodi LJ (1931) Huesos de los miembros de los macroquénidos neoterciarios. Physis 10:294–304. https://doi.org/10.1002/pmic.201500224
Pascual R, Jaureguizar EO (1990) Evolving climates and mammal faunas in cenozoic South America. J Hum Evol 19:23–60. https://doi.org/10.1016/0047-2484(90)90011-Y
Pascual R, Cattoi N V, Francis JC, et al (1966) Paleontografía Bonaerense, Fascículo IV, Vertebrata. Comisión de Investigación Científica, La Plata
Pascual R, Ortiz Jaureguizar E, Prado JL (1996) Land mammals: Paradigm for Cenozoic South American geobiotic evolution. Münch Geowissen Abhand (A) 30:265–319
Pascual R, Vucetich MG, Scillato-Yané GJ, Bond M (1985) Main pathways of mammalian diversification in South America. In: Stehli FG, Webb SD (eds) The Great American Biotic Interchange. Springer, Dordrecht, pp 219–239
Paula Couto C (1978) Ungulados fosseis do Riochiquense de Itaborai, RJ, Brasil. I - Xenungulata. An Acad Bras Cienc 50:203–207
Perea D, Martínez S (2004) Estratigrafía del Mioceno-Pleistoceno en el litoral sur-oeste de Uruguay. In: Veroslavsky G, Ubilla M, Martínez S (eds) Cuencas Sedimentarias de Uruguay: Geología, Paleontología y Recursos Naturales – Cenozoico. DIRAC, Montevideo, pp 105–124
Perea D, Rinderknecht A, Ubilla M, et al (2013) Mamíferos y estratigrafía del Neógeno de Uruguay. In: Brandoni D, Noriega JI (eds) El Neógeno de la Mesopotamia Argentina. Asociación Paleontológica Argentina, Publicación Especial 14, pp 192–206
Polly PD (2007) Limbs in mammalian evolution. In: Hall BK (ed) Fins into Limbs: Evolution, Development, and Transformation. University of Chicago Press, Chicago, pp 245–268
Pound MJ, Haywood AM, Salzmann U, et al (2011) A Tortonian (Late Miocene, 11.61-7.25Ma) global vegetation reconstruction. Palaeogeogr Palaeoclimatol Palaeoecol 300:29–45. https://doi.org/10.1016/j.palaeo.2010.11.029
Pound MJ, Haywood AM, Salzmann U, Riding JB (2012) Global vegetation dynamics and latitudinal temperature gradients during the Mid to Late Miocene (15.97-5.33Ma). Earth Sci Rev 112:1–22. https://doi.org/10.1016/j.earscirev.2012.02.005
Prado JL, Martinez-Maza C, Alberdi MT (2015) Megafauna extinction in South America: A new chronology for the Argentine Pampas. Palaeogeogr Palaeoclimatol Palaeoecol 425:41–49. https://doi.org/10.1016/j.palaeo.2015.02.026
Prevosti FJ, Forasiepi A, Zimicz N (2013) The evolution of the Cenozoic terrestrial mammalian predator guild in South America: competition or replacement? J Mamm Evol 20:3–21. https://doi.org/10.1007/s10914-011-9175-9
Prevosti FJ, Forasiepi AM (2018) Evolution of South American Mammalian Predators During the Cenozoic: Paleobiogeographic and Paleoenvironmental Contingencies. Springer Geology, Cham, Switzerland
Prevosti FJ, Romano CO, Forasiepi AM, et al (2021) New radiometric 40Ar-39Ar dates and faunistic analyses refine evolutionary dynamics of Neogene vertebrate assemblages in southern South America. Sci Rep 11:1–14. https://doi.org/10.1038/s41598-021-89135-1
Prothero DR, Campbell KE, Beatty BL, Frailey CD (2014) New late Miocene dromomerycine artiodactyl from the Amazon Basin: implications for interchange dynamics. J Paleontol 88:434–443. https://doi.org/10.1666/13-022
Püschel HP, O’Reilly JE, Pisani D, Donoghue PCJ (2020) The impact of fossil stratigraphic ranges on tip-calibration, and the accuracy and precision of divergence time estimates. Palaeontology 63:67–83. https://doi.org/10.1111/pala.12443
Pyron RA (2017) Novel approaches for phylogenetic inference from morphological data and total-evidence dating in squamate reptiles (Lizards, Snakes, and Amphisbaenians). Syst Biol 66:38–56. https://doi.org/10.1093/sysbio/syw068
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Rech JA, Currie BS, Jordan TE, et al (2019) Massive middle Miocene gypsic paleosols in the Atacama Desert and the formation of the Central Andean rain-shadow. Earth Planet Sci Lett 506:184–194. https://doi.org/10.1016/j.epsl.2018.10.040
Rojo MA (1985) Un aporte al conocimiento del Terciario marino: Formación Bahía Inglesa. In: IV Congreso Geológico Chileno. Antofagasta, Chile, pp 514–533
Ronquist F, Teslenko M, Van Der Mark P, et al (2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542. https://doi.org/10.1093/sysbio/sys029
Rose KD (2006) The Beginning of the Age of Mammals. The Johns Hopkins University Press, Baltimore
Rose KD, Holbrook LT, Kumar K, et al (2019) Anatomy, relationships, and paleobiology of Cambaytherium (Mammalia, Perissodactylamorpha, Anthracobunia) from the lower Eocene of western India. J Vertebr Paleontol 39:1–147. https://doi.org/10.1080/02724634.2020.1761370
Rovereto C (1914) Los estratos araucanos y sus fósiles. An Mus Nac Hist Nat Buenos Aires 25:1–250
Rusconi C (1932) Nuevos restos de Scalabrinitherium del Terciario de Paraná y apuntes relativos a su anatomía craneana. Rev Med Vet 15–19 2–6:3–18
Rusconi C (1957) Evolución de la trompa en las macrauquenias. Rev Mus Hist Nat Mendoza 10:111–118
Salas-Gismondi R, Ochoa D, Jouve S, et al (2022) Miocene fossils from the southeastern Pacific shed light on the last radiation of marine crocodylians. Proc R Soc B Biol Sci 289:20220380. https://doi.org/10.1098/rspb.2022.0380
Schindelin J, Arganda-Carreras I, Frise E, et al (2012) Fiji: An open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Schmidt GI (2013) Litopterna y Notoungulata (Mammalia) de la Formación Ituzaingó (Mioceno tardío) de la provincia de Entre Ríos: sistemática, bioestratigrafía y paleobiogeografía. Dissertation, Universidad Nacional de La Plata, Buenos Aires, Argentina
Schmidt GI, Ferrero BS (2014) Taxonomic reinterpretation of Theosodon hystatus Cabrera and Kraglievich, 1931 (Litopterna, Macraucheniidae) and phylogenetic relationships of the family. J Vertebr Paleontol 34:1231–1238. https://doi.org/10.1080/02724634.2014.837393
Schmidt GI, Montalvo CI, Cerdeño E, et al (2022) Updated data on Litopterna from the Huayquerian Stage/Age (Late Miocene-Early Pliocene) of central-east Argentina. C R Palevol 21:721–743
Scott KM (1990) Postcranial dimensions of ungulates as predictors of body mass. In: Damuth J, MacFadden BJ (eds) Body Size in Mammalian Paleobiology: Estimation and Biological Implications. Cambridge University Press, Cambridge, UK, pp 301–377
Scott WB (1910) Mammalia of the Santa Cruz beds: Litopterna. In: Scott WB (ed) Reports of the Princeton University Expeditions to Patagonia, 1896-1899, vol 7, Paleontology 4. Princeton University, E. Schweizerbart’sche Verlashandlung. Stuttgart, pp 1–156
Scott WB (1912) Toxodonta and Entelonychia of the Santa Cruz Beds. In: Scott WB (ed) Reports of the Princeton University Expeditions to Patagonia, 1896–1899, vol. 6, Paleontology 3. Mammalia of the Santa Cruz Beds, part 2–3. Princeton University, E. Schweizerbart’sche Verlashandlung. Stuttgart, pp 111–300
Sefve I (1925) Macrauchenia patagonica. Bull Geol Inst Univ Upsala 19:1–21.
Seiffert ER, Tejedor MF, Fleagle JG, et al (2020) A parapithecid stem anthropoid of African origin in the Paleogene of South America. Science 368:194–197. https://doi.org/10.1126/science.aba1135
Shelley SL, Williamson TE, Brusatte SL (2018) The osteology of Periptychus carinidens: A robust, ungulate-like placental mammal (Mammalia: Periptychidae) from the Paleocene of North America. PLoS One 13:e0200132. https://doi.org/10.1371/journal.pone.0200132
Shockey BJ (1999) Postcranial osteology and functional morphology of the Litopterna of Salla, Bolivia (Late Oligocene). J Vertebr Paleontol 19:383–390. https://doi.org/10.1080/02724634.1999.10011149
Simpson GG (1945) The principles of classification and a classification of mammals. Bull Am Mus Nat Hist 85:1–350
Simpson GG (1948) The beginning of the age of mammals in South America, Part 1. Bull Am Mus Nat Hist 91:1–232. https://doi.org/10.1007/s10914-010-9153-7
Simpson GG (1980) Splendid Isolation: the Curious History of South American Mammals. Yale University Press, New Haven
Sinclair WJ (1909) Typotheria of the Santa Cruz Beds. In: Scott WB (ed) Reports of the Princeton University Expeditions to Patagonia, 1896–1899, vol. 6, Paleontology. Mammalia of the Santa Cruz Beds, part 1. Princeton University, E. Schweizerbart’sche Verlashandlung. Stuttgart, pp 1–110
Sisson S (1914) The Anatomy of the Domestic Animals. W. B. Saunders Company, Philadelphia
Soibelzon E, Soibelzon LH, Gasparini GM, Tonni EP (2019) El Pleistoceno de la provincia de Buenos Aires y sus mamíferos. Opera Lilloana 52:606–637
Soria MF (1981) Los Litopterna del Colhuehuapense (Oligoceno Tardío) de la Argentina. Rev Mus Argent Nat "Bernardino Rivadavia" III:1–54
Soria MF (1986) Huayqueriana Kraglievich, 1934, género de Macraucheniidae (Litopterna) de edad Huayqueriense (Mioceno tardío).Aspectos evolutivos vinculados. In: IV Congreso Argentino de Paleontología y Bioestratigrafía, Actas, pp 157–164
Soria MF (2001) Los Proterotheriidae (Litopterna, Mammalia), sistemática, origen y filogenia. Monogr Mus Argent Cienc Nat 1:1–167
Soto-Acuña S, Otero RA, Rubilar-Rogers D, Vargas AO (2015) Arcosaurios no avianos de Chile. In: Rubilar-Rogers D, Otero R, Vargas A, Sallaberry M (eds) Vertebrados Fósiles de Chile, Publicación Ocasional del Museo Nacional de Historia Natural, N° 63, pp 209–263
Standring S (2015) Gray’s Anatomy, the Anatomical Basis of Clinical Practice, 41st Ed. Elsevier
Stein BR, Casinos A (1997) What is a cursorial mammal? J Zool 242:185–192. https://doi.org/10.1111/j.1469-7998.1997.tb02939.x
Suárez ME, Lamilla J, Marquardt C (2004) Peces Chimaeriformes (Chondrichthyes, Holocephali) del Neógeno de la Formación Bahía Inglesa (Región de Atacama, Chile). Rev Geol Chile 31:105–114. https://doi.org/10.4067/s0716-02082004000100006
Taylor ME (1974) The functional anatomy of the forelimb of some African Viverridae (Carnivora). J Morphol 143:307–335. https://doi.org/10.1002/jmor.1051480208
Tejada-Lara J V., Salas-Gismondi R, Pujos F, et al (2015) Life in proto-Amazonia: Middle Miocene mammals from the Fitzcarrald Arch (Peruvian Amazonia). Palaeontology 58:341–378. https://doi.org/10.1111/pala.12147
Tonni EP (1990) Mamíferos del Holoceno en la provincia de Buenos Aires. Paula-Coutiana 4:3–21
Vizcaíno SF, Cassini GH, Toledo N, Bargo S (2012) On the evolution of large size in mammalian herbivores of Cenozoic faunas of southern South America. In: Patterson B, Costa LP (eds) Bones, Clones, and Biomes: The History and Geography of Recent Neotropical Mammals. The University of Chicago Press, Chicago, pp 76–101
Walsh SA, Suárez M (2005) First post−Mesozoic record of Crocodyliformes from Chile. Acta Palaeontol Pol 50:595–600
Welker F, Collins MJ, Thomas JA, et al (2015) Ancient proteins resolve the evolutionary history of Darwin’s South American ungulates. Nature 522:81–84. https://doi.org/10.1038/nature14249
Westbury M, Baleka S, Barlow A, et al (2017) A mitogenomic timetree for Darwin’s enigmatic South American mammal Macrauchenia patachonica. Nat Commun 8:1–8. https://doi.org/10.1038/ncomms15951
Wood AR, Bebej RM, Manz CL, et al (2011) Postcranial functional morphology of Hyracotherium (Equidae, Perissodactyla) and locomotion in the earliest horses. J Mamm Evol 18:1–32. https://doi.org/10.1007/s10914-010-9145-7
Woodburne MO (2010) The Great American Biotic Interchange: dispersals, tectonics, climate, sea level and holding pens. J Mamm Evol 17:245–264. https://doi.org/10.1007/s10914-010-9144-8
Woodburne MO, Goin FJ, Bond M, et al (2014a) Paleogene land mammal faunas of South America; a response to global climatic changes and indigenous floral diversity. J Mamm Evol 21:1–73. https://doi.org/10.1007/s10914-012-9222-1
Woodburne MO, Goin FJ, Raigemborn MS, et al (2014a) Revised timing of the South American early Paleogene land mammal ages. J South Am Earth Sci 54:109–119. https://doi.org/10.1016/j.jsames.2014.05.003
Zachos J, Pagani H, Sloan L, et al (2001) Trends, rhythms, and aberrations in global climate 65 Ma to present. Science 292:686–693. https://doi.org/10.1126/science.1059412
Acknowledgements
In a world in the midst of a pandemic, conducting this research was particularly challenging, and it would have been extremely difficult without the generous support from colleagues. We would like to thank Gabriela Schmidt, Leonardo Lobo, Andy McGrath, Alejandro Kramarz, Óscar Rojas Mondaca (MUHNCAL-KM), Pip Brewer (NHMUK), and Bárbara Vera for sharing photographs/3D models/old publications of important specimens that improved the anatomical comparisons of this study. We are particularly grateful to Agustín Martinelli, who even took new photographs from the MACN collections to assist us in this study. H.P.P. also thanks Gabriela Schmidt for the interesting discussions about dental anatomy in macraucheniids. We thank the curators of the following institutions for their kind assistance during museum the visits of H.P.P.: Daniel Brinkman and Vanessa Rhue (YPM VPPU), Agustín Martinelli (MACN), Laura Chornogubsky (MACN), Guillaume Billet (MNHN), Marcelo Reguero (MLP), Susana Bargo (MLP), Gabriel Aguirre (PIMUZ), Marcelo Sanchez-Villagra (PIMUZ), and David Rubilar-Rogers (SGO.PV). We also thank Gabriela Schmidt, an anonymous reviewer, and the editors, François Pujos and Darin Croft, for helpful comments that improved this manuscript.
Funding
This research was funded by the National Agency for Research and Development (ANID)/PFCHA/Doctorado en el extranjero Becas Chile/2018-72190003; the European Research Council (ERC) starting grant PalM, under the European Union’s Horizon 2020 Research and Innovation Programme (no. 756226) to H.P.P. S.L.S. and S.L.B. J.A.-M. and S.S-A. are supported for ANID scholarships for PhD studies in Chile.
Author information
Authors and Affiliations
Contributions
H.P.P. conceived and designed the study. J.A.-M. and S.S.-A prepared SGO.PV.21700 and took the final pictures of the specimen. H.P.P. with the assistance of J.A.-M. and R. U. prepared the figures. R.U. provided the stratigraphic context of the SGO.PV.21700. H.P.P. examined SGO.PV.21700 anatomy, took the specimens measurements, conducted the phylogenetic analyses and wrote an initial manuscript. H.P.P, J.A.-M., S.S.-A, R.U., S.L.S, and S.L.B interpreted the obtained results and contributed to the writing of the submitted version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Püschel, H.P., Alarcón-Muñoz, J., Soto-Acuña, S. et al. Anatomy and phylogeny of a new small macraucheniid (Mammalia: Litopterna) from the Bahía Inglesa Formation (late Miocene), Atacama Region, Northern Chile. J Mammal Evol 30, 415–460 (2023). https://doi.org/10.1007/s10914-022-09646-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10914-022-09646-0